Kinetic studies and homology modeling of a dual-substrate linalool/nerolidol synthase from Plectranthus amboinicus
- PMID: 34429465
- PMCID: PMC8385045
- DOI: 10.1038/s41598-021-96524-z
Kinetic studies and homology modeling of a dual-substrate linalool/nerolidol synthase from Plectranthus amboinicus
Abstract
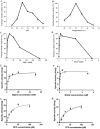
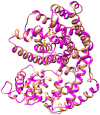
Linalool and nerolidol are terpene alcohols that occur naturally in many aromatic plants and are commonly used in food and cosmetic industries as flavors and fragrances. In plants, linalool and nerolidol are biosynthesized as a result of respective linalool synthase and nerolidol synthase, or a single linalool/nerolidol synthase. In our previous work, we have isolated a linalool/nerolidol synthase (designated as PamTps1) from a local herbal plant, Plectranthus amboinicus, and successfully demonstrated the production of linalool and nerolidol in an Escherichia coli system. In this work, the biochemical properties of PamTps1 were analyzed, and its 3D homology model with the docking positions of its substrates, geranyl pyrophosphate (C10) and farnesyl pyrophosphate (C15) in the active site were constructed. PamTps1 exhibited the highest enzymatic activity at an optimal pH and temperature of 6.5 and 30 °C, respectively, and in the presence of 20 mM magnesium as a cofactor. The Michaelis-Menten constant (Km) and catalytic efficiency (kcat/Km) values of 16.72 ± 1.32 µM and 9.57 × 10-3 µM-1 s-1, respectively, showed that PamTps1 had a higher binding affinity and specificity for GPP instead of FPP as expected for a monoterpene synthase. The PamTps1 exhibits feature of a class I terpene synthase fold that made up of α-helices architecture with N-terminal domain and catalytic C-terminal domain. Nine aromatic residues (W268, Y272, Y299, F371, Y378, Y379, F447, Y517 and Y523) outlined the hydrophobic walls of the active site cavity, whilst residues from the RRx8W motif, RxR motif, H-α1 and J-K loops formed the active site lid that shielded the highly reactive carbocationic intermediates from the solvents. The dual substrates use by PamTps1 was hypothesized to be possible due to the architecture and residues lining the catalytic site that can accommodate larger substrate (FPP) as demonstrated by the protein modelling and docking analysis. This model serves as a first glimpse into the structural insights of the PamTps1 catalytic active site as a multi-substrate linalool/nerolidol synthase.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants in Biotechnology of isoprenoids (eds. Schrader, J. & Bohlmann, J.) 63-106 (Springer, 2015). - PubMed
-
- George, K. W., Alonso-Gutierrez, J., Keasling, J. D. & Lee, T. S. Isoprenoid drugs, biofuels, and chemicals—artemisinin, farnesene, and beyond in Biotechnology of isoprenoids (eds. Schrader, J. & Bohlmann, J.) 355–389 (Springer, 2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

