Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles
- PMID: 34429860
- PMCID: PMC8363910
- DOI: 10.1002/jev2.12134
Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles
Abstract
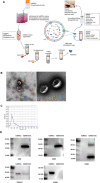
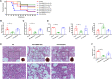
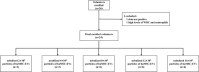
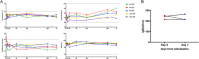
Mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) turn out to be a promising source of cell-free therapy. Here, we investigated the biodistribution and effect of nebulized human adipose-derived MSC-EVs (haMSC-EVs) in the preclinical lung injury model and explored the safety of nebulized haMSC-EVs in healthy volunteers. DiR-labelled haMSC-EVs were used to explore the distribution of nebulized haMSC-EVs in the murine model. Pseudomonas aeruginosa-induced murine lung injury model was established, and survival rate, as well as WBC counts, histology, IL-6, TNF-α and IL-10 levels in bronchoalveolar lavage fluid (BALF) were measured to explore the optimal therapeutic dose of haMSC-EVs through the nebulized route. Twenty-four healthy volunteers were involved and received the haMSC-EVs once, ranging from 2 × 108 particles to 16 × 108 particles (MEXVT study, NCT04313647). Nebulizing haMSC-EVs improved survival rate to 80% at 96 h in P. aeruginosa-induced murine lung injury model by decreasing lung inflammation and histological severity. All volunteers tolerated the haMSC-EVs nebulization well, and no serious adverse events were observed from starting nebulization to the 7th day after nebulization. These findings suggest that nebulized haMSC-EVs could be a promising therapeutic strategy, offering preliminary evidence to promote the future clinical applications of nebulized haMSC-EVs in lung injury diseases.
Keywords: extracellular vesicles; healthy volunteers; lung injury; mesenchymal stromal cells; nebulization.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures

References
-
- Bartolucci, J., Verdugo, F. J., González, P. L., Larrea, R. E., Abarzua, E., Goset, C., Rojo, P., Palma, I., Lamich, R., Pedreros, P. A., Valdivia, G., Lopez, V. M., Nazzal, C., Alcayaga‐Miranda, F., Cuenca, J., Brobeck, M. J., Patel, A. N., Figueroa, F. E., & Khoury, M. (2017).Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal. Circulation Research, 121, 1192–1204. - PMC - PubMed
-
- Camussi, G., Deregibus, M. C., Bruno, S., Cantaluppi, V., & Biancone, L.. (2010). Exosomes/microvesicles as a mechanism of cell‐to‐cell communication. Kidney International 78, 838–848. - PubMed
-
- Curley, G. F., Jerkic, M., Dixon, S., Hogan, G., Masterson, C., O'Toole, D., Devaney, J., & Laffey, J. G. (2017). Cryopreserved, Xeno‐free human umbilical cord mesenchymal stromal cells reduce lung injury severity and bacterial burden in rodent Escherichia coli‐induced acute respiratory distress syndrome. Critical Care Medicine 45, e202–e212. - PubMed
-
- Devaney, J., Horie, S., Masterson, C., Elliman, S., Barry, F., O'Brien, T., Curley, G. F., O'Toole, D., & Laffey, J. G. (2015).Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax, 70, 625–635. - PubMed
-
- Dinh, P‐U. C., Paudel, D., Brochu, H., Popowski, K. D., Gracieux, M. C., Cores, J., Huang, K., Hensley, M. T., Harrell, E., Vandergriff, A. C., George, A. K., Barrio, R. T., Hu, S., Allen, T. A., Blackburn, K., Caranasos, T. G., Peng, X., Schnabel, L. V., Adler, K. B.…Cheng, K. (2020).Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nature communications, 11, 1064. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

