Macronutrient balancing in free-ranging populations of moose
- PMID: 34429914
- PMCID: PMC8366896
- DOI: 10.1002/ece3.7909
Macronutrient balancing in free-ranging populations of moose
Abstract
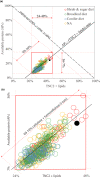
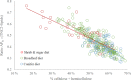
At northern latitudes, large spatial and temporal variation in the nutritional composition of available foods poses challenges to wild herbivores trying to satisfy their nutrient requirements. Studies conducted in mostly captive settings have shown that animals from a variety of taxonomic groups deal with this challenge by adjusting the amounts and proportions of available food combinations to achieve a target nutrient balance. In this study, we used proportions-based nutritional geometry to analyze the nutritional composition of rumen samples collected in winter from 481 moose (Alces alces) in southern Sweden and examine whether free-ranging moose show comparable patterns of nutrient balancing. Our main hypothesis was that wild moose actively regulate their rumen nutrient composition to offset ecologically imposed variation in the nutritional composition of available foods. To test this, we assessed the macronutritional composition (protein, carbohydrates, and lipids) of rumen contents and commonly eaten foods, including supplementary feed, across populations with contrasting winter diets, spanning an area of approximately 10,000 km2. Our results suggest that moose balanced the macronutrient composition of their rumen, with the rumen contents having consistently similar proportional relationship between protein and nonstructural carbohydrates, despite differences in available (and eaten) foods. Furthermore, we found that rumen macronutrient balance was tightly related to ingested levels of dietary fiber (cellulose and hemicellulose), such that the greater the fiber content, the less protein was present in the rumen compared with nonstructural carbohydrates. Our results also suggest that moose benefit from access to a greater variety of trees, shrubs, herbs, and grasses, which provides them with a larger nutritional space to maneuver within. Our findings provide novel theoretical insights into a model species for ungulate nutritional ecology, while also generating data of direct relevance to wildlife and forest management, such as silvicultural or supplementary feeding practices.
Keywords: Alces alces; deer; herbivory; nutritional ecology; primate; ungulate.
© 2021 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Aharoni, Y., & Tagari, H. (1991). Use of N‐15 determinations of purine nitrogen fraction of digesta to define nitrogen‐metabolism traits in the rumen. Journal of Dairy Science, 74, 2540–2547. - PubMed
-
- Allen, M. S. (1997). Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. Journal of Dairy Science, 80, 1447–1462. - PubMed
-
- Asher, G. W., Stevens, D. R., Archer, J. A., Barrel, G. K., Scott, I. C., Ward, J. F., & Littlejohn, R. P. (2011). Energy and protein as nutritional drivers of lactation and calf growth of farmed red deer. Livestock Science, 140, 8–16.
-
- Barboza, P. S., Parker, K. L., & Hume, I. D. (2008). Integrative wildlife nutrition. Springer Science & Business Media.
-
- Belovsky, G. E. (1978). Diet optimization in a generalist herbivore ‐ Moose. Theoretical Population Biology, 14, 105–134. - PubMed
Associated data
LinkOut - more resources
Full Text Sources

