A molecular based method for rapid detection of Salmonella spp. in poultry dust samples
- PMID: 34430257
- PMCID: PMC8374386
- DOI: 10.1016/j.mex.2021.101356
A molecular based method for rapid detection of Salmonella spp. in poultry dust samples
Abstract
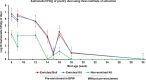
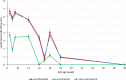
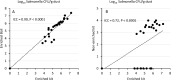
Salmonellosis, caused by Salmonella spp., is a widely reported foodborne zoonosis frequently associated with ingestion of poultry products. Salmonella vaccination of chickens can be used to reduce bacterial shedding and risk of human infection. To determine Salmonella burden in chicken farms, culture methods of environmental samples that require a turn-around time of 5-7 days are usually used. Rapid screening using molecular assays such as PCR of pre-enriched broth has been reported for Salmonella spp. detection in feed, floor dust, and drag swabs within 2-3 days. Here we report an adaptation of the method for detection of Salmonella in poultry dust samples collected using a settle plate method under experimental conditions. Key features:•Passive dust sample collection using dry settle plates without media suspended from dropper lines of drinkers.•Small amount of sample required for the pre-enrichment process.•Quantification of Salmonella DNA with high sensitivity using an inexpensive extraction protocol.
Keywords: Chicken; Disease monitoring; Poultry dust; Salmonella; qPCR.
© 2021 The Author(s). Published by Elsevier B.V.
Figures

References
-
- Cerquetti M.C., Gherardi M.M. Vaccination of chickens with a temperature-sensitive mutant of Salmonella enteritidis. Vaccine. 2000;18(11-12):1140–1145. - PubMed
-
- Desin T.S., Köster W., Potter A.A. Salmonella vaccines in poultry: past, present and future. Expert Rev. Vaccines. 2013;12(1):87–96. - PubMed
-
- Gole V.C. Shedding of Salmonella in single age caged commercial layer flock at an early stage of lay. Int. J. Food Microbiol. 2014;189:61–66. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

