Porous tantalum structure integrated on Ti6Al4V base by Laser Powder Bed Fusion for enhanced bony-ingrowth implants: In vitro and in vivo validation
- PMID: 34430760
- PMCID: PMC8367833
- DOI: 10.1016/j.bioactmat.2021.05.025
Porous tantalum structure integrated on Ti6Al4V base by Laser Powder Bed Fusion for enhanced bony-ingrowth implants: In vitro and in vivo validation
Abstract
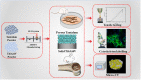
Despite the widespread application of Ti6Al4V and tantalum (Ta) in orthopedics, bioinertia and high cost limit their further applicability, respectively, and tremendous efforts have been made on the Ti6Al4V-Ta alloy and Ta coating to address these drawbacks. However, the scaffolds obtained are unsatisfactory. In this study, novel high-interface-strength Ti6Al4V-based porous Ta scaffolds were successfully manufactured using Laser Powder Bed Fusion for the first time, in which porous Ta was directly manufactured on a solid Ti6Al4V substrate. Mechanical testing revealed that the novel scaffolds were biomechanically compatible, and the interfacial bonding strength was as high as 447.5 MPa. In vitro biocompatibility assay, using rat bone marrow mesenchymal stem cells (r-BMSCs), indicated that the novel scaffolds were biocompatible. Alkaline phosphatase and mineralized nodule determination demonstrated that the scaffolds favored the osteogenic differentiation of r-BMSCs. Moreover, scaffolds were implanted into rabbits with femur bone defects, and imaging and histological evaluation identified considerable new bone formation and bone ingrowth, suggesting that the scaffolds were well integrated with the host bone. Overall, these results demonstrated good mechanical compatibility, biocompatibility, and osteointegration performance of the novel Ti6Al4V-based porous Ta scaffold, which possesses great potential for orthopedic clinical applications.
Keywords: Laser powder bed fusion; Orthopedic scaffolds; Osteointegration; Tantalum; Ti6Al4V.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






References
-
- Zadpoor A.A. Meta-biomaterials. Biomater. Sci. 2019;8:18–38. - PubMed
-
- Kaur M., Singh K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Materials science & engineering C, Materials for biological applications. 2019;102:844–862. - PubMed
-
- Wauthle R., Ahmadi S.M., Amin Yavari S. Revival of pure titanium for dynamically loaded porous implants using additive manufacturing. Materials science & engineering C, Materials for biological applications. 2015;54:94–100. - PubMed
-
- Han Q., Wang C., Chen H. Porous tantalum and titanium in orthopedics: a review. ACS Biomater. Sci. Eng. 2019;5:5798–5824. - PubMed
LinkOut - more resources
Full Text Sources

