HIV self-testing with digital supports as the new paradigm: A systematic review of global evidence (2010-2021)
- PMID: 34430835
- PMCID: PMC8367787
- DOI: 10.1016/j.eclinm.2021.101059
HIV self-testing with digital supports as the new paradigm: A systematic review of global evidence (2010-2021)
Abstract
Background: HIV self-testing (HIVST) is recommended by the WHO as an innovative strategy to reach UNAIDS targets to end HIV by 2030. HIVST with digital supports is defined as the use of digital interventions (e.g., website-based, social media, mobile HIVST applications (apps), text messaging (SMS), digital vending machines (digital VMs)) to improve the efficiency and impact of HIVST. HIVST deployment and integration in health services is an emerging priority. We conducted a systematic review aiming to close the gap in evidence that summarizes the impact of digitally supported HIVST and to inform policy recommendations.
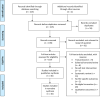
Methods: We searched PubMed and Embase for articles and abstracts on HIVST with digital supports published during the period February 1st, 2010 to June 15th, 2021, following Cochrane guidelines and PRISMA methodology. We assessed feasibility, acceptability, preference, and impact outcomes across all populations and study designs. Metrics reported were willingness to use HIVST, preferences for HIVST delivery, proportion of first-time testers, HIVST uptake, HIVST kit return rate, and linkage to care. Heterogeneity of the interventions and reported metrics precluded us from conducting a meta-analysis.
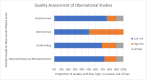
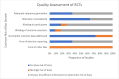
Findings: 46 studies were narratively synthesized, of which 72% were observational and 28% were RCTs. Half of all studies (54%, 25/46) assessed web-based innovations (e.g., study websites, videos, chatbots), followed by social media (26%, 12/46), HIVST-specific apps (7%, 3/46), SMS (9%, 4/46), and digital VMs (4%, 2/46). Web-based innovations were found to be acceptable (77-97%), preferred over in-person and hybrid options by more first-time testers (47-48%), highly feasible (93-95%), and were overall effective in supporting linkage to care (53-100%). Social media and app-based innovations also had high acceptability (87-95%) and linkage to care proportions (80-100%). SMS innovations increased kit return rates (54-94%) and HIVST uptake among hard-to-reach groups. Finally, digital VMs were highly acceptable (54-93%), and HIVST uptake was six times greater when using digital VMs compared to distribution by community workers.
Interpretation: HIVST with digital supports was deemed feasible, acceptable, preferable, and was shown to increase uptake, engage first-time testers and hard-to-reach populations, and successfully link participants to treatment. Findings pave the way for greater use of HIVST interventions with digital supports globally.
Keywords: Digital; HIV; Intervention; Mhealth; Online; Self-testing.
© 2021 The Author(s).
Conflict of interest statement
Dr Pant Pai reports a copyright for HIVSmart! (an open access mobile application), issued by McGill University (#1105598) in 2013, and co-owned by Grand Challenges Canada and McGill University. The other authors have no conflicts of interest to declare.
Figures

References
-
- World Health Organization. Number of countries adopting HIV self-testing policies rises sharply. July 25, 2017. https://www.who.int/hiv/mediacentre/news/hiv-self-testing-increases/en/#... (accessed May 10, 2021).
-
- World Health Organization. Innovative WHO HIV testing recommendations aim to expand treatment coverage. November 27, 2019. https://www.who.int/news/item/27-11-2019-innovative-who-hiv-testing-reco.... (accessed May 10, 2021).
-
- UNAIDS. UNAIDS report on the global AIDS epidemic shows that 2020 targets will not be met because of deeply unequal success; COVID-19 risks blowing HIV progress way off course. July 6, 2020. https://www.unaids.org/en/resources/presscentre/pressreleaseandstatement.... (accessed May 10, 2021).
-
- World Health Organization. Monitoring and evaluating digital health interventions: a practical guide to conducting research and assessment. 2016. https://saluddigital.com/wp-content/uploads/2019/06/WHO.-Monitoring-and-.... (accessed May 10, 2021).
-
- Roehr B. FDA approves “instant” HIV home test. BMJ. 2012;345:e4636. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

