Evaluation of procalcitonin-guided antimicrobial stewardship in patients admitted to hospital with COVID-19 pneumonia
- PMID: 34430872
- PMCID: PMC8378277
- DOI: 10.1093/jacamr/dlab133
Evaluation of procalcitonin-guided antimicrobial stewardship in patients admitted to hospital with COVID-19 pneumonia
Abstract
Background: Procalcitonin is a biomarker that may be able to identify patients with COVID-19 pneumonia who do not require antimicrobials for bacterial respiratory tract co-infections.
Objectives: To evaluate the safety and effectiveness of a procalcitonin-guided algorithm in rationalizing empirical antimicrobial prescriptions in non-critically ill patients with COVID-19 pneumonia.
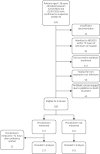
Methods: Retrospective, single-site, cohort study in adults hospitalized with confirmed or suspected COVID-19 pneumonia and receiving empirical antimicrobials for potential bacterial respiratory tract co-infection. Regression models were used to compare the following outcomes in patients with and without procalcitonin testing within 72 h of starting antimicrobials: antimicrobial consumption (DDD); antimicrobial duration; a composite safety outcome of death, admission to HDU/ICU or readmission to hospital within 30 days; and length of admission. Procalcitonin levels of ≤0.25 ng/L were interpreted as negatively predictive of bacterial co-infection. Effects were expressed as ratios of means (ROM) or prevalence ratios (PR) accordingly.
Results: 259 patients were included in the final analysis. Antimicrobial use was lower in patients who had procalcitonin measured within 72 h of starting antimicrobials: mean antimicrobial duration 4.4 versus 5.4 days, adjusted ROM 0.7 (95% CI 0.6-0.9); mean antimicrobial consumption 6.8 versus 8.4 DDD, adjusted ROM 0.7 (95% CI 0.6-0.8). Both groups had similar composite safety outcomes (adjusted PR 0.9; 95% CI 0.6-1.3) and lengths of admission (adjusted ROM 1.3; 95% CI 0.9-1.6).
Conclusions: A procalcitonin-guided algorithm may allow for the safe reduction of antimicrobial usage in hospitalized non-critically ill patients with COVID-19 pneumonia.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures

References
-
- Pritchard M, Dankwa EA, Hall M. et al. ISARIC Clinical Data Report 4 October 2020. medRxiv.2020. http://medrxiv.org/content/early/2020/10/06/2020.07.17.20155218.abstract.
-
- Zhang JJ, Dong X, Cao YY. et al.Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; 75: 1730–41. - PubMed
-
- ISARIC (International Severe Acute Respiratory and Emerging Infections Consortium). COVID-19 Report. 2020. https://isaric.tghn.org.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
