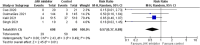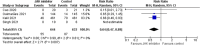Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials
- PMID: 34431004
- PMCID: PMC8384394
- DOI: 10.1007/s10067-021-05884-4
Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials
Abstract
Coronavirus disease-2019 (COVID-19) represents a global public health nightmare. The "cytokine storm," the most prominent underlying pathophysiologic mechanism of this disease, can theoretically be targeted at several stages. Janus kinase (JAK) inhibitors constitute a drug class that could ameliorate the inflammatory response and enhance antibody production. Herein, we aimed to evaluate the efficacy of JAK inhibitors in patients with COVID-19, performing the most updated relevant meta-analysis. We searched two major databases for randomized controlled trials (RCTs) enrolling adult patients with documented COVID-19 in the in-hospital setting, assigned either to JAK inhibitor treatment plus standard of care or standard of care alone. We set as primary efficacy outcome the endpoint of COVID-19 death on day 28 and as secondary efficacy composite outcome that of mechanical ventilation or initiation of extracorporeal membrane oxygenation (ECMO). We finally pooled data of interest from 4 RCTs in a total of 1338 subjects with documented COVID-19 infection, utilizing the following JAK inhibitors: baricitinib, ruxolitinib, tofacitinib, and nezulcitinib. Treatment with JAK inhibitor compared to control resulted in a significant reduction in the risk for COVID-19 death by 43%, while it also led to a significant decrease in the risk for mechanical ventilation or ECMO initiation by 36%. Herein, we demonstrate a clear benefit with JAK inhibitors added to standard of care in patients with COVID-19 in terms of risk reduction concerning major outcomes. Larger RCTs will elucidate their place in treatment armamentarium against COVID-19.
Keywords: COVID-19; Janus kinase inhibitor; Mechanical ventilation; Mortality.
© 2021. International League of Associations for Rheumatology (ILAR).
Figures
References
-
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo-Hernandez H, Rochwerg B, Lamontagne F, Han MA, Liu Q, Agarwal A, Agoritsas T, Chu DK, Couban R, Darzi A, Devji T, Fang B, Fang C, Flottorp SA, Foroutan F, Ghadimi M, Heels-Ansdell D, Honarmand K, Hou L, Hou X, Ibrahim Q, Khamis A, Lam B, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Qasim A, Rada G, Riaz IB, Sadeghirad B, Sekercioglu N, Sheng L, Sreekanta A, Switzer C, Tendal B, Thabane L, Tomlinson G, Turner T, Vandvik PO, Vernooij RW, Viteri-García A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello-Petersen R, Qasim A, Martinez JPD, Cusano E. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ. 2020;371:m4852. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical