Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept
- PMID: 34431207
- PMCID: PMC9906354
- DOI: 10.1111/ajt.16814
Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept
Abstract
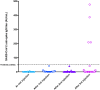
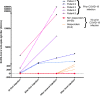
Poor responses to mRNA COVID-19 vaccine have been reported after 2 vaccine injections in kidney transplant recipients (KTRs) treated with belatacept. We analyzed the humoral response in belatacept-treated KTRs without a history of SARS-CoV-2 infection who received three injections of BNT162b2-mRNA COVID-19 vaccine. We also investigated vaccine immunogenicity in belatacept-treated KTRs with prior COVID-19 and characterized symptomatic COVID-19 infections after the vaccine in belatacept-treated KTRs. Among the 62 belatacept-treated KTRs (36 [58%] males), the median age (63.5 years IQR [51-72]), without COVID-19 history, only four patients (6.4%) developed anti-SARS-CoV-2 IgG with low antibody titers (median 209, IQR [20-409] AU/ml). 71% were treated with mycophenolic acid and 100% with steroids in association with belatacept. In contrast, in all the 5 KTRs with prior COVID-19 history, mRNA vaccine induced a strong antibody response with high antibody titers (median 10 769 AU/ml, IQR [6410-20 069]) after two injections. Seroprevalence after three-vaccine doses in 35 non-belatacept-treated KTRs was 37.1%. Twelve KTRs developed symptomatic COVID-19 after vaccination, including severe forms (50% of mortality). Breakthrough COVID-19 occurred in 5% of fully vaccinated patients. Administration of a third dose of BNT162b2 mRNA COVID-19 vaccine did not improve immunogenicity in KTRs treated with belatacept without prior COVID-19. Other strategies aiming to improve patient protection are needed.
Keywords: COVID-19 mRNA vaccine; belatacept; kidney transplant recipients; three doses.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

