Halogen-Induced Chemical Injury to the Mammalian Cardiopulmonary Systems
- PMID: 34431415
- PMCID: PMC8807065
- DOI: 10.1152/physiol.00004.2021
Halogen-Induced Chemical Injury to the Mammalian Cardiopulmonary Systems
Abstract
The halogens chlorine (Cl2) and bromine (Br2) are highly reactive oxidizing elements with widespread industrial applications and a history of development and use as chemical weapons. When inhaled, depending on the dose and duration of exposure, they cause acute and chronic injury to both the lungs and systemic organs that may result in the development of chronic changes (such as fibrosis) and death from cardiopulmonary failure. A number of conditions, such as viral infections, coexposure to other toxic gases, and pregnancy increase susceptibility to halogens significantly. Herein we review their danger to public health, their mechanisms of action, and the development of pharmacological agents that when administered post-exposure decrease morbidity and mortality.
Keywords: ARDS; antioxidants; bromine; chlorine; heme.
Figures


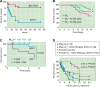
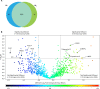
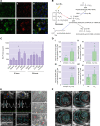
References
-
- Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA, ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly. An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness. Ann Am Thorac Soc 14: 1060–1072, 2017. doi:10.1513/AnnalsATS.201704-297WS. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

