LLF580, an FGF21 Analog, Reduces Triglycerides and Hepatic Fat in Obese Adults With Modest Hypertriglyceridemia
- PMID: 34431493
- PMCID: PMC8914500
- DOI: 10.1210/clinem/dgab624
LLF580, an FGF21 Analog, Reduces Triglycerides and Hepatic Fat in Obese Adults With Modest Hypertriglyceridemia
Abstract
Purpose: To evaluate the safety and potential efficacy of LLF580, a genetically engineered variant of human fibroblast growth factor-21, for triglyceride lowering, weight loss, and hepatic fat reduction.
Methods: A multicenter, double-blind, parallel design trial in obese, mildly hypertriglyceridemic adults randomized (1:1) to LLF580 300 mg or placebo subcutaneously every 4 weeks for 3 doses.
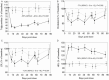
Results: Of 64 randomized study participants, 61 (mean ± SD: age 45 ± 11 years, 49% male, 80/15/5% Caucasian/African American/other, body mass index 36.1 ± 3.8 kg/m2) received LLF580 (n = 30) or placebo (n = 31) at 7 research sites in the United States. LLF580 lowered serum triglycerides by 54% (least square mean placebo adjusted change from baseline), total cholesterol 7%, low-density lipoprotein cholesterol 12%, and increased high-density lipoprotein cholesterol 36% compared with placebo (all P < 0.001) over 12 weeks. Substantial reduction of liver fat of 52% over placebo (P < 0.001) was also demonstrated in the setting of improved liver function tests including alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, the composite enhanced liver fibrosis score, and N-terminal type III collagen propeptide (all P < 0.05). Insulin and C-peptide levels and insulin resistance by homeostatic model assessment for insulin resistance were all lower, and adiponectin higher with LLF580 treatment compared with placebo, whereas fasting glucose and glycated hemoglobin were unchanged. Reductions in biomarkers of bone formation without differences in markers of bone resorption were observed. LLF580 was generally safe and well tolerated, except for higher incidence of generally mild to moderate gastrointestinal adverse effects.
Conclusions: In obese, mildly hypertriglyceridemic adults, LLF580 was generally safe and demonstrated beneficial effects on serum lipids, liver fat, and biomarkers of liver injury, suggesting it may be effective for treatment of select metabolic disorders including hypertriglyceridemia and nonalcoholic fatty liver disease. Assessments of longer term safety and efficacy are warranted.
Clinicaltrials.gov identifier: NCT03466203.
Keywords: fibroblast growth factor 21 (FGF21); hypertriglyceridemia; metabolism; nonalcoholic fatty liver diseases (NAFLD); obesity.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Another Kid on the Block: Long-acting FGF21 Analogue to Treat Dyslipidemia and Fatty Liver.J Clin Endocrinol Metab. 2022 Jan 1;107(1):e417-e419. doi: 10.1210/clinem/dgab686. J Clin Endocrinol Metab. 2022. PMID: 34529079 Free PMC article. No abstract available.
References
-
- Kharitonenkov A, Dunbar JD, Bina HA, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215(1):1-7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

