Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations
- PMID: 34431689
- PMCID: PMC8552704
- DOI: 10.1128/Spectrum.00062-21
Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations
Abstract
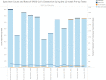
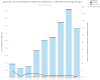
The coronavirus disease 2019 (COVID-19) pandemic has challenged clinical diagnostic operations due to supply shortages and high staffing needs to collect nasopharyngeal (NP) swab samples. Saliva is an easily accessible alternative specimen type to overcome some of these challenges. In this study, we first used paired saliva and NP swab specimens (n = 128) to compare test performance characteristics with three RNA extraction platforms, i.e., Maxwell RSC (Promega), MagNA Pure 96 (Roche), and KingFisher Flex (Thermo Fisher Scientific), together with two PCR chemistries, i.e., severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2019-nCoV) Centers for Disease Control and Prevention (CDC) quantitative PCR (qPCR) probe assay (Integrated DNA Technologies) and TagPath COVID-19 combination kit (Thermo Fisher Scientific). This study demonstrated that both saliva and NP swab specimens performed well, with 97% agreement when tested by the CDC qPCR chemistry using Maxwell and MagNA Pure RNA extraction platforms. We then compared 12 weeks of saliva and NP swab testing results using two independent asymptomatic populations, including a community surveillance program using saliva samples only (n = 466) and preoperative screening using NP swab samples only (n = 8,461). The positive detection rates among participants with either saliva or NP swab samples were 1.07% and 1.12%, respectively, which confirms the low pretest probabilities for COVID-19 infections in asymptomatic populations. Notably, there was no increased proportion of low-titer cases (inconclusive results) reported in the asymptomatic groups, compared with the all-comers groups (0.21% and 0.66%, respectively, in the community population and 0.25% and 0.49%, respectively, in the preoperative population); this suggests that low-viral-titer carriers can be found similarly in both groups with saliva or NP swab specimens. In summary, saliva can be considered a good alternative for noninvasive but well-instructed self-collection. IMPORTANCE Our study shows that saliva is a noninvasive respiratory secretion sample type that contains equal or more host materials (RNase P), compared with those contained in the corresponding NP swab specimens, in 103 paired samples. SARS-CoV-2 detection with two RNA extraction platforms, Maxwell and MagNA Pure, with CDC qPCR chemistry showed similar test sensitivities for paired specimens. We then analyzed SARS-CoV-2 detections rates in two independent groups of asymptomatic participants, i.e., a group at a community screening station with supervised saliva collection only (n = 466) and a preoperative screening group (n = 8,461) with NP swabbing only. Similar detection rates of 1.07% for the community group and 1.12% for the preoperative group supported the similar test performances in these groups predicted to have low pretest probabilities of infection. With mindful preparation, saliva can be considered for schools and clinical participants when adequate collection education can be provided and compliance can be established.
Keywords: SARS-CoV-2; asymptomatic; saliva.
Figures


References
-
- American Society for Microbiology. 2021. Supply shortages impacting COVID-19 and non-COVID testing. https://asm.org/Articles/2020/September/Clinical-Microbiology-Supply-Sho....
-
- Butler-Laporte G, Lawandi A, Schiller I, Yao M, Dendukuri N, McDonald EG, Lee TC. 2021. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med 181:353–360. doi: 10.1001/jamainternmed.2020.8876. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

