Interleukin 6 and Cardiovascular Outcomes in Patients With Chronic Kidney Disease and Chronic Coronary Syndrome
- PMID: 34431970
- PMCID: PMC8387946
- DOI: 10.1001/jamacardio.2021.3079
Interleukin 6 and Cardiovascular Outcomes in Patients With Chronic Kidney Disease and Chronic Coronary Syndrome
Abstract
Importance: Inflammation promotes cardiovascular disease and anti-inflammatory treatment reduces cardiovascular events in patients with chronic coronary syndrome. Chronic kidney disease (CKD) is a risk factor for cardiovascular disease. It is unclear how inflammation mediated by interleukin 6 (IL-6) in patients with CKD is linked to cardiovascular disease.
Objective: To investigate associations between IL-6 and cardiovascular outcomes in patients with chronic coronary syndrome in association with kidney function.
Design, setting, and participants: This multicenter cohort study included patients enrolled at 663 centers in 39 countries with chronic coronary syndrome who were included in the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial. Patients were enrolled between December 2008 and April 2010 and were followed up for a median length of 3.7 years. Analysis in this substudy began September 2020.
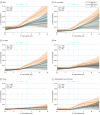
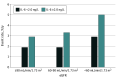
Exposures: Exposures were IL-6 and creatinine estimated glomerular filtration rates (eGFR), which were collected at baseline. Associations between continuous and categorical levels (<2.0 ng/L vs ≥2.0 ng/L) of IL-6 and cardiovascular outcomes were tested in association with eGFR cutoffs (normal eGFR level [≥90 mL/min/1.73 m2], mildly decreased eGFR level [60-90 mL/min/1.73 m2], and moderately to severely decreased eGFR level [<60 mL/min/1.73 m2]).
Main outcomes and measures: Main outcome was major adverse cardiovascular events (MACE), a composite of cardiovascular death, myocardial infarction, and stroke.
Results: This substudy of the STABILITY trial included 14 611 patients with available IL-6 levels at baseline. The median (interquartile range) age was 65 (59-71) years, and 2700 (18.5%) were female. During follow-up, MACE occurred in 1459 individuals (10.0%). Higher levels of IL-6 were in continuous models independently associated with risk of MACE (P < .001) in all CKD strata. Using predefined strata, elevated IL-6 level (≥2.0 vs <2.0 ng/L) was associated with increased risk of MACE at normal kidney function (2.9% vs 1.9% events/y [hazard ratio, 1.35; 95% CI, 1.02-1.78]), mild CKD (3.3% vs 1.9% [hazard ratio, 1.57; 95% CI, 1.35-1.83]), and moderate to severe CKD (5.0% vs 2.9% [hazard ratio, 1.60; 95% CI, 1.28-1.99]).
Conclusions and relevance: In patients with chronic coronary syndrome, elevated levels of IL-6 were associated with risk of MACE in all CKD strata. Thus, IL-6 and CKD stage may help when identifying patients with chronic coronary syndrome for anti-inflammatory treatment.
Conflict of interest statement
Figures
References
-
- Held C, White HD, Stewart RAH, et al. ; STABILITY Investigators . Inflammatory biomarkers interleukin-6 and c-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. 2017;6(10):e005077. doi: 10.1161/JAHA.116.005077 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

