Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of GROINSS-V II
- PMID: 34432481
- PMCID: PMC8577685
- DOI: 10.1200/JCO.21.00006
Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of GROINSS-V II
Abstract
Purpose: The Groningen International Study on Sentinel nodes in Vulvar cancer (GROINSS-V)-II investigated whether inguinofemoral radiotherapy is a safe alternative to inguinofemoral lymphadenectomy (IFL) in vulvar cancer patients with a metastatic sentinel node (SN).
Methods: GROINSS-V-II was a prospective multicenter phase-II single-arm treatment trial, including patients with early-stage vulvar cancer (diameter < 4 cm) without signs of lymph node involvement at imaging, who had primary surgical treatment (local excision with SN biopsy). Where the SN was involved (metastasis of any size), inguinofemoral radiotherapy was given (50 Gy). The primary end point was isolated groin recurrence rate at 24 months. Stopping rules were defined for the occurrence of groin recurrences.
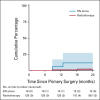
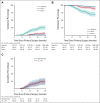
Results: From December 2005 until October 2016, 1,535 eligible patients were registered. The SN showed metastasis in 322 (21.0%) patients. In June 2010, with 91 SN-positive patients included, the stopping rule was activated because the isolated groin recurrence rate in this group went above our predefined threshold. Among 10 patients with an isolated groin recurrence, nine had SN metastases > 2 mm and/or extracapsular spread. The protocol was amended so that those with SN macrometastases (> 2 mm) underwent standard of care (IFL), whereas patients with SN micrometastases (≤ 2 mm) continued to receive inguinofemoral radiotherapy. Among 160 patients with SN micrometastases, 126 received inguinofemoral radiotherapy, with an ipsilateral isolated groin recurrence rate at 2 years of 1.6%. Among 162 patients with SN macrometastases, the isolated groin recurrence rate at 2 years was 22% in those who underwent radiotherapy, and 6.9% in those who underwent IFL (P = .011). Treatment-related morbidity after radiotherapy was less frequent compared with IFL.
Conclusion: Inguinofemoral radiotherapy is a safe alternative for IFL in patients with SN micrometastases, with minimal morbidity. For patients with SN macrometastasis, radiotherapy with a total dose of 50 Gy resulted in more isolated groin recurrences compared with IFL.
Conflict of interest statement
Figures


Comment in
-
[Radiotherapy versus inguinofemoral lymphadenectomy in vulvar cancer patients with micrometastases in the sentinel node].Strahlenther Onkol. 2022 Jun;198(6):595-597. doi: 10.1007/s00066-022-01922-5. Epub 2022 Apr 12. Strahlenther Onkol. 2022. PMID: 35415784 Free PMC article. German. No abstract available.
References
-
- Van der Zee AG, Oonk MH, De Hullu JA, et al. : Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 26:884-889, 2008 - PubMed
-
- Pouwer AW, Hinten F, van der Velden J, et al. : Volume-controlled versus short drainage after inguinofemoral lymphadenectomy in vulvar cancer patients: A Dutch nationwide prospective study. Gynecol Oncol 146:580-587, 2017 - PubMed
-
- Oonk MH, van Hemel BM, Hollema H, et al. : Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early-stage vulvar cancer: Results from GROINSS-V, a multicentre observational study. Lancet Oncol 11:646-652, 2010 - PubMed
-
- Taylor A, Rockall AG, Powell ME: An atlas of the pelvic lymph node regions to aid radiotherapy target volume definition. Clin Oncol (R Coll Radiol) 19:542-550, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

