Statins Are Associated With Increased Insulin Resistance and Secretion
- PMID: 34433298
- PMCID: PMC8551023
- DOI: 10.1161/ATVBAHA.121.316159
Statins Are Associated With Increased Insulin Resistance and Secretion
Abstract
Objective: Statin treatment reduces the risk of atherosclerotic cardiovascular disease but is associated with a modest increased risk of type 2 diabetes, especially in those with insulin resistance or prediabetes. Our objective was to determine the physiological mechanism for the increased type 2 diabetes risk.
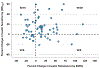
Approach and results: We conducted an open-label clinical trial of atorvastatin 40 mg daily in adults without known atherosclerotic cardiovascular disease or type 2 diabetes at baseline. The co-primary outcomes were changes at 10 weeks versus baseline in insulin resistance as assessed by steady-state plasma glucose during the insulin suppression test and insulin secretion as assessed by insulin secretion rate area under the curve (ISRAUC) during the graded-glucose infusion test. Secondary outcomes included glucose and insulin, both fasting and during oral glucose tolerance test. Of 75 participants who enrolled, 71 completed the study (median age 61 years, 37% women, 65% non-Hispanic White, median body mass index, 27.8 kg/m2). Atorvastatin reduced LDL (low-density lipoprotein)-cholesterol (median decrease 53%, P<0.001) but did not change body weight. Compared with baseline, atorvastatin increased insulin resistance (steady-state plasma glucose) by a median of 8% (P=0.01) and insulin secretion (ISRAUC) by a median of 9% (P<0.001). There were small increases in oral glucose tolerance test glucoseAUC (median increase, 0.05%; P=0.03) and fasting insulin (median increase, 7%; P=0.01).
Conclusions: In individuals without type 2 diabetes, high-intensity atorvastatin for 10 weeks increases insulin resistance and insulin secretion. Over time, the risk of new-onset diabetes with statin use may increase in individuals who become more insulin resistant but are unable to maintain compensatory increases in insulin secretion.
Trial registration: ClinicalTrials.gov NCT02437084.
Keywords: atorvastatin; cardiovascular disease; glucose; insulin resistance; insulin secretion.
Conflict of interest statement
Disclosures
The authors declare that there is no conflict of interest.
Figures

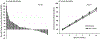

Comment in
-
Statin-Related New-Onset Diabetes Appears Driven by Increased Insulin Resistance: Are There Clinical Implications?Arterioscler Thromb Vasc Biol. 2021 Nov;41(11):2798-2801. doi: 10.1161/ATVBAHA.121.316893. Epub 2021 Oct 27. Arterioscler Thromb Vasc Biol. 2021. PMID: 34705475 No abstract available.
References
-
- Collins R, Reith C, Emberson J et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. - PubMed
-
- Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–3209. - PubMed
-
- Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr., et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

