Primary Chemoablation of Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer Using UGN-102, a Mitomycin-Containing Reverse Thermal Gel (Optima II): A Phase 2b, Open-Label, Single-Arm Trial
- PMID: 34433303
- PMCID: PMC8667793
- DOI: 10.1097/JU.0000000000002186
Primary Chemoablation of Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer Using UGN-102, a Mitomycin-Containing Reverse Thermal Gel (Optima II): A Phase 2b, Open-Label, Single-Arm Trial
Abstract
Purpose: Low-grade intermediate-risk nonmuscle-invasive bladder cancer (LG IR NMIBC) is a recurrent disease, thus requiring repeated transurethral resection of bladder tumor under general anesthesia. We evaluated the efficacy and safety of UGN-102, a mitomycin-containing reverse thermal gel, as a primary chemoablative therapeutic alternative to transurethral resection of bladder tumor for patients with LG IR NMIBC.
Materials and methods: This prospective, phase 2b, open-label, single-arm trial recruited patients with biopsy-proven LG IR NMIBC to receive 6 once-weekly instillations of UGN-102. The primary end point was complete response (CR) rate, defined as the proportion of patients with negative endoscopic examination, negative cytology and negative for-cause biopsy 3 months after treatment initiation. Patients with CR were followed quarterly up to 12 months to assess durability of treatment effect. Safety and adverse events were monitored throughout the trial.
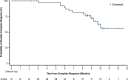
Results: A total of 63 patients (38 males and 25 females 33-96 years old) enrolled and received ≥1 instillation of UGN-102. Among the patients 41 (65%) achieved CR at 3 months, of whom 39 (95%), 30 (73%) and 25 (61%) remained disease-free at 6, 9 and 12 months after treatment initiation, respectively. A total of 13 patients had documented recurrences. The probability of durable response 9 months after CR (12 months after treatment initiation) was estimated to be 73% by Kaplan-Meier analysis. Common adverse events (incidence ≥10%) included dysuria, urinary frequency, hematuria, micturition urgency, urinary tract infection and fatigue.
Conclusions: Nonsurgical primary chemoablation of LG IR NMIBC using UGN-102 resulted in significant treatment response with sustained durability. UGN-102 may provide an alternative to repetitive surgery for patients with LG IR NMIBC.
Trial registration: ClinicalTrials.gov NCT03558503.
Keywords: clinical trial; mitomycin; phase II; urinary bladder neoplasms.
Conflict of interest statement
JL, DM, JDR and DSa report acting as investigators and consultants/advisors for UroGen Pharma; KC, BH, NDS and ABS report serving as consultants/advisors for UroGen Pharma; NG, SL, MM, AM, SR, MS and ES are full-time employees of UroGen Pharma; MT reports serving as an assistant editor for
Figures
Comment in
-
Editorial Comment.J Urol. 2022 Jan;207(1):69. doi: 10.1097/JU.0000000000002186.01. Epub 2021 Oct 18. J Urol. 2022. PMID: 34661456 No abstract available.
-
Editorial Comment.J Urol. 2022 Jan;207(1):69. doi: 10.1097/JU.0000000000002186.02. Epub 2021 Oct 18. J Urol. 2022. PMID: 34661457 No abstract available.
References
-
- American Cancer Society (ACS): Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020.
-
- Babjuk M, Burger M, Comperat EM, et al. : European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol 2019; 76: 639. - PubMed
-
- Monteiro LL, Witjes JA, Agarwal PK, et al. : ICUD-SIU International Consultation on Bladder Cancer 2017: management of non-muscle invasive bladder cancer. World J Urol 2019; 37: 51. - PubMed
-
- Chang SS, Boorjian SA, Chou R, et al. : Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol 2016; 196: 1021. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical