Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R+/- mouse model of ALSP, which can be rescued via CSF1R inhibitors
- PMID: 34433559
- PMCID: PMC8386924
- DOI: 10.1126/sciadv.abg1601
Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R+/- mouse model of ALSP, which can be rescued via CSF1R inhibitors
Abstract
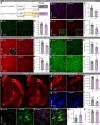
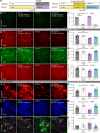
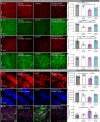
Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia is an autosomal dominant neurodegenerative disease caused by mutations in colony-stimulating factor 1 receptor (CSF1R). We sought to identify the role of microglial CSF1R haploinsufficiency in mediating pathogenesis. Using an inducible Cx3cr1 CreERT2/+-Csf1r +/fl system, we found that postdevelopmental, microglia-specific Csf1r haploinsufficiency resulted in reduced expression of homeostatic microglial markers. This was associated with loss of presynaptic surrogates and the extracellular matrix (ECM) structure perineuronal nets. Similar phenotypes were observed in constitutive global Csf1r haploinsufficient mice and could be reversed/prevented by microglia elimination in adulthood. As microglial elimination is unlikely to be clinically feasible for extended durations, we treated adult CSF1R+/- mice at different disease stages with a microglia-modulating dose of the CSF1R inhibitor PLX5622, which prevented microglial dyshomeostasis along with synaptic- and ECM-related deficits. These data highlight microglial dyshomeostasis as a driver of pathogenesis and show that CSF1R inhibition can mitigate these phenotypes.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Oyanagi K., Kinoshita M., Suzuki-Kouyama E., Inoue T., Nakahara A., Tokiwai M., Arai N., Satoh J. I., Aoki N., Jinnai K., Yazawa I., Arai K., Ishihara K., Kawamura M., Ishizawa K., Hasegawa K., Yagisita S., Amano N., Yoshida K., Terada S., Yoshida M., Akiyama H., Mitsuyama Y., Ikeda S. I., Adult onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) and Nasu-Hakola disease: Lesion staging and dynamic changes of axons and microglial subsets. Brain Pathol. 27, 748–769 (2017). - PMC - PubMed
-
- C. Sundal, Z. K. Wszolek, CSF1R-related adult-onset leukoencephalopathy with axonal spheroids and pigmented glia, in GeneReviews®, M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. H. Bean, G. Mirzaa, A. Amemiya, Eds. (University of Washington, 1993). - PubMed
-
- Hiyoshi M., Hashimoto M., Yukihara M., Bhuyan F., Suzu S., M-CSF receptor mutations in hereditary diffuse leukoencephalopathy with spheroids impair not only kinase activity but also surface expression. Biochem. Biophys. Res. Commun. 440, 589–593 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

