Vimseltinib: A Precision CSF1R Therapy for Tenosynovial Giant Cell Tumors and Diseases Promoted by Macrophages
- PMID: 34433663
- PMCID: PMC9398179
- DOI: 10.1158/1535-7163.MCT-21-0361
Vimseltinib: A Precision CSF1R Therapy for Tenosynovial Giant Cell Tumors and Diseases Promoted by Macrophages
Abstract
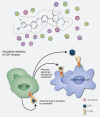
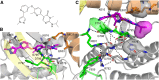
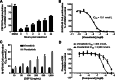
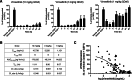
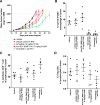
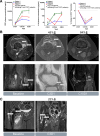
Macrophages can be co-opted to contribute to neoplastic, neurologic, and inflammatory diseases. Colony-stimulating factor 1 receptor (CSF1R)-dependent macrophages and other inflammatory cells can suppress the adaptive immune system in cancer and contribute to angiogenesis, tumor growth, and metastasis. CSF1R-expressing osteoclasts mediate bone degradation in osteolytic cancers and cancers that metastasize to bone. In the rare disease tenosynovial giant cell tumor (TGCT), aberrant CSF1 expression and production driven by a gene translocation leads to the recruitment and growth of tumors formed by CSF1R-dependent inflammatory cells. Small molecules and antibodies targeting the CSF1/CSF1R axis have shown promise in the treatment of TGCT and cancer, with pexidartinib recently receiving FDA approval for treatment of TGCT. Many small-molecule kinase inhibitors of CSF1R also inhibit the closely related kinases KIT, PDGFRA, PDGFRB, and FLT3, thus CSF1R suppression may be limited by off-target activity and associated adverse events. Vimseltinib (DCC-3014) is an oral, switch control tyrosine kinase inhibitor specifically designed to selectively and potently inhibit CSF1R by exploiting unique features of the switch control region that regulates kinase conformational activation. In preclinical studies, vimseltinib durably suppressed CSF1R activity in vitro and in vivo, depleted macrophages and other CSF1R-dependent cells, and resulted in inhibition of tumor growth and bone degradation in mouse cancer models. Translationally, in a phase I clinical study, vimseltinib treatment led to modulation of biomarkers of CSF1R inhibition and reduction in tumor burden in TGCT patients.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
- p. 2095
References
-
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. . Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors.Cancer Res 2007;67:2649–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

