Lipid-based and protein-based interactions synergize transmembrane signaling stimulated by antigen clustering of IgE receptors
- PMID: 34433665
- PMCID: PMC8536334
- DOI: 10.1073/pnas.2026583118
Lipid-based and protein-based interactions synergize transmembrane signaling stimulated by antigen clustering of IgE receptors
Abstract
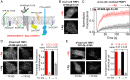
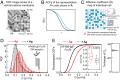
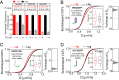
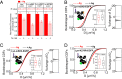
Antigen (Ag) crosslinking of immunoglobulin E-receptor (IgE-FcεRI) complexes in mast cells stimulates transmembrane (TM) signaling, requiring phosphorylation of the clustered FcεRI by lipid-anchored Lyn tyrosine kinase. Previous studies showed that this stimulated coupling between Lyn and FcεRI occurs in liquid ordered (Lo)-like nanodomains of the plasma membrane and that Lyn binds directly to cytosolic segments of FcεRI that it initially phosphorylates for amplified activity. Net phosphorylation above a nonfunctional threshold is achieved in the stimulated state but not in the resting state, and current evidence supports the hypothesis that this relies on Ag crosslinking to disrupt a balance between Lyn and tyrosine phosphatase activities. However, the structural interactions that underlie the stimulation process remain poorly defined. This study evaluates the relative contributions and functional importance of different types of interactions leading to suprathreshold phosphorylation of Ag-crosslinked IgE-FcεRI in live rat basophilic leukemia mast cells. Our high-precision diffusion measurements by imaging fluorescence correlation spectroscopy on multiple structural variants of Lyn and other lipid-anchored probes confirm subtle, stimulated stabilization of the Lo-like nanodomains in the membrane inner leaflet and concomitant sharpening of segregation from liquid disordered (Ld)-like regions. With other structural variants, we determine that lipid-based interactions are essential for access by Lyn, leading to phosphorylation of and protein-based binding to clustered FcεRI. By contrast, TM tyrosine phosphatase, PTPα, is excluded from these regions due to its Ld-preference and steric exclusion of TM segments. Overall, we establish a synergy of lipid-based, protein-based, and steric interactions underlying functional TM signaling in mast cells.
Keywords: FcεRI; Imaging FCS; plasma membrane domains; rafts; transmembrane signaling.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Sigalov A., Multi-chain immune recognition receptors: Spatial organization and signal transduction. Semin. Immunol. 17, 51–64 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

