An in vitro vesicle formation assay reveals cargo clients and factors that mediate vesicular trafficking
- PMID: 34433667
- PMCID: PMC8536394
- DOI: 10.1073/pnas.2101287118
An in vitro vesicle formation assay reveals cargo clients and factors that mediate vesicular trafficking
Abstract
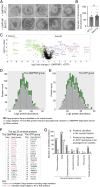
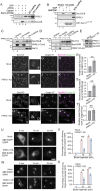
The fidelity of protein transport in the secretory pathway relies on the accurate sorting of proteins to their correct destinations. To deepen our understanding of the underlying molecular mechanisms, it is important to develop a robust approach to systematically reveal cargo proteins that depend on specific sorting machinery to be enriched into transport vesicles. Here, we used an in vitro assay that reconstitutes packaging of human cargo proteins into vesicles to quantify cargo capture. Quantitative mass spectrometry (MS) analyses of the isolated vesicles revealed cytosolic proteins that are associated with vesicle membranes in a GTP-dependent manner. We found that two of them, FAM84B (also known as LRAT domain containing 2 or LRATD2) and PRRC1, contain proline-rich domains and regulate anterograde trafficking. Further analyses revealed that PRRC1 is recruited to endoplasmic reticulum (ER) exit sites, interacts with the inner COPII coat, and its absence increases membrane association of COPII. In addition, we uncovered cargo proteins that depend on GTP hydrolysis to be captured into vesicles. Comparing control cells with cells depleted of the cargo receptors, SURF4 or ERGIC53, we revealed specific clients of each of these two export adaptors. Our results indicate that the vesicle formation assay in combination with quantitative MS analysis is a robust and powerful tool to uncover novel factors that mediate vesicular trafficking and to uncover cargo clients of specific cellular factors.
Keywords: COPII; cargo receptor; cargo sorting; intracellular protein transport; secretory pathway.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Guo Y., Sirkis D. W., Schekman R., Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 30, 169–206 (2014). - PubMed
-
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R., Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20, 87–123 (2004). - PubMed
-
- Dancourt J., Barlowe C., Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 79, 777–802 (2010). - PubMed
-
- Nichols W. C., et al. ., Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 93, 61–70 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

