Infections at the maternal-fetal interface: an overview of pathogenesis and defence
- PMID: 34433930
- PMCID: PMC8386341
- DOI: 10.1038/s41579-021-00610-y
Infections at the maternal-fetal interface: an overview of pathogenesis and defence
Abstract
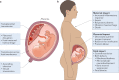
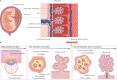
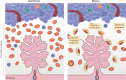
Infections are a major threat to human reproductive health, and infections in pregnancy can cause prematurity or stillbirth, or can be vertically transmitted to the fetus leading to congenital infection and severe disease. The acronym 'TORCH' (Toxoplasma gondii, other, rubella virus, cytomegalovirus, herpes simplex virus) refers to pathogens directly associated with the development of congenital disease and includes diverse bacteria, viruses and parasites. The placenta restricts vertical transmission during pregnancy and has evolved robust mechanisms of microbial defence. However, microorganisms that cause congenital disease have likely evolved diverse mechanisms to bypass these defences. In this Review, we discuss how TORCH pathogens access the intra-amniotic space and overcome the placental defences that protect against microbial vertical transmission.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Christianson, A., Howson, C. & Modell, B. March of Dimes. Global Report on Birth Defect. The Hidden toll of Dying and Disabled Children (March of Dimes Birth Defects Foundation, 2006).
-
- Lawn JE, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

