NF-κB: At the Borders of Autoimmunity and Inflammation
- PMID: 34434197
- PMCID: PMC8381650
- DOI: 10.3389/fimmu.2021.716469
NF-κB: At the Borders of Autoimmunity and Inflammation
Abstract
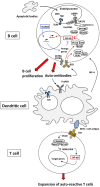
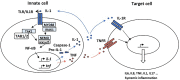
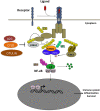
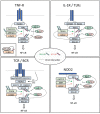
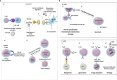
The transcription factor NF-κB regulates multiple aspects of innate and adaptive immune functions and serves as a pivotal mediator of inflammatory response. In the first part of this review, we discuss the NF-κB inducers, signaling pathways, and regulators involved in immune homeostasis as well as detail the importance of post-translational regulation by ubiquitination in NF-κB function. We also indicate the stages of central and peripheral tolerance where NF-κB plays a fundamental role. With respect to central tolerance, we detail how NF-κB regulates medullary thymic epithelial cell (mTEC) development, homeostasis, and function. Moreover, we elaborate on its role in the migration of double-positive (DP) thymocytes from the thymic cortex to the medulla. With respect to peripheral tolerance, we outline how NF-κB contributes to the inactivation and destruction of autoreactive T and B lymphocytes as well as the differentiation of CD4+-T cell subsets that are implicated in immune tolerance. In the latter half of the review, we describe the contribution of NF-κB to the pathogenesis of autoimmunity and autoinflammation. The recent discovery of mutations involving components of the pathway has both deepened our understanding of autoimmune disease and informed new therapeutic approaches to treat these illnesses.
Keywords: autoinflammation; genetic diseases; NF-κB; autoimmunity; immune tolerance; ubiquitination.
Copyright © 2021 Barnabei, Laplantine, Mbongo, Rieux-Laucat and Weil.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

