Septaly Oriented Mild Aortic Regurgitant Jets Negatively Influence Left Ventricular Blood Flow-Insights From 4D Flow MRI Animal Study
- PMID: 34434980
- PMCID: PMC8380779
- DOI: 10.3389/fcvm.2021.711099
Septaly Oriented Mild Aortic Regurgitant Jets Negatively Influence Left Ventricular Blood Flow-Insights From 4D Flow MRI Animal Study
Abstract
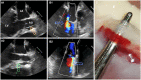
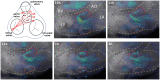
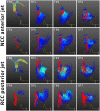
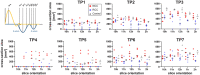
Objectives: Paravalvular leakage (PVL) and eccentric aortic regurgitation remain a major clinical concern in patients receiving transcatheter aortic valve replacement (TAVR), and regurgitant volume remains the main readout parameter in clinical assessment. In this work we investigate the effect of jet origin and trajectory of mild aortic regurgitation on left ventricular hemodynamics in a porcine model. Methods: A pig model of mild aortic regurgitation/PVL was established by transcatheter piercing and dilating the non-coronary (NCC) or right coronary cusp (RCC) of the aortic valve close to the valve annulus. The interaction between regurgitant blood and LV hemodynamics was assessed by 4D flow cardiovascular MRI. Results: Six RCC, six NCC, and two control animals were included in the study and with one dropout in the NCC group, the success rate of model creation was 93%. Regurgitant jets originating from NCC were directed along the ventricular side of the anterior mitral leaflet and integrated well into the diastolic vortex forming in the left ventricular outflow tract. However, jets from the RCC were orientated along the septum colliding with flow within the vortex, and progressing down to the apex. As a consequence, the presence as well as the area of the vortex was reduced at the site of impact compared to the NCC group. Impairment of vortex formation was localized to the area of impact and not the entire vortex ring. Blood from the NCC jet was largely ejected during the following systole, whereas ejection of large portion of RCC blood was protracted. Conclusions: Even for mild regurgitation, origin and trajectory of the regurgitant jet does cause a different effect on LV hemodynamics. Septaly oriented jets originating from RCC collide with the diastolic vortex, reduce its size, and reach the apical region of the left ventricle where blood resides extendedly. Hence, RCC jets display hemodynamic features which may have a potential negative impact on the long-term burden to the heart.
Keywords: 4D flow MRI; aortic regurgitation; intraventricular hemodynamics; mild regurgitation; paravalvular leakage; translational large animal model; vortex formation.
Copyright © 2021 Cesarovic, Weisskopf, Kron, Glaus, Peper, Buoso, Suendermann, Canic, Falk, Kozerke, Emmert and Stoeck.
Conflict of interest statement
VF has relevant (institutional) financial activities outside the submitted work with following commercial entities: Medtronic GmbH, Biotronik SE & Co., Abbott GmbH & Co. KG, Boston Scientific, Edwards Lifesciences, Berlin Heart, Novartis Pharma GmbH, JOTEC/CryoLife GmbH, Zurich Heart. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kodali S, Pibarot P, Douglas PS, Williams M, Xu K, Thourani V, et al. . Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. (2015) 36:449–lpage>56. 10.1093/eurheartj/ehu384 - DOI - PubMed
-
- Kampaktsis PN, Subramayam P, Sherifi I, Vavuranakis M, Siasos G, Tousoulis D, et al. . Impact of paravalvular leak on left ventricular remodeling and global longitudinal strain 1 year after transcatheter aortic valve replacement. Future Cardiol. (2021) 17:337–lpage>45. 10.2217/fca-2020-0086 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

