The Importance of Appropriate Dosing of Nonvitamin K Antagonist Oral Anticoagulants for Stroke Prevention in Patients with Atrial Fibrillation
- PMID: 34435170
- PMCID: PMC8382498
- DOI: 10.1055/s-0041-1731777
The Importance of Appropriate Dosing of Nonvitamin K Antagonist Oral Anticoagulants for Stroke Prevention in Patients with Atrial Fibrillation
Abstract
Preventing thromboembolic events, while minimizing bleeding risks, remains challenging when managing patients with atrial fibrillation (AF). Several factors contribute to current dosing patterns of nonvitamin K antagonist oral anticoagulants (NOACs), including patient characteristics, comorbidities, and physician judgment. Application of NOAC doses inconsistent with the drug labels may cause patients to receive either subtherapeutic (increasing stroke risk) or supratherapeutic (increasing bleeding risk) anticoagulant levels. In clinical practice, under- or over-dosing of NOACs in patients with AF is not uncommon. This analysis of prospective and retrospective registry and database studies on NOAC use in patients with AF (with at least 250 patients in each treatment arm) showed that under-dosing may be associated with reduced effectiveness for stroke prevention, with similar or even increased bleeding than with the standard dose. This may reflect underlying conditions and patient factors that increase bleeding despite NOAC dose reduction. Such factors could drive the observed overuse of reduced NOAC dosages, often making the prescription of reduced-dose NOAC an intentional label deviation. In contrast, over-dosing more likely occurs accidentally; instead of providing benefits, it may be associated with worse safety outcomes than the standard dose, including increased bleeding risk and higher all-cause mortality rates. This review summarizes the main findings on NOAC doses usually prescribed to patients with AF in clinical practice.
Keywords: anticoagulants; atrial fibrillation; stroke.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. ( https://creativecommons.org/licenses/by/4.0/ ).
Conflict of interest statement
Conflict of Interest J.B-.W. has received honoraria from Alexion, Bayer, Daiichi Sankyo, Medscape, Pfizer, and Portola, and research funding from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, and Portola. M.F. has received funding from Abbott, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Dawn 4S, INRStar, Medtronic, Oberoi Consulting, Pfizer, Roche, Sanofi Aventis, and Servier. W.A. has served as an advisor or consultant for Biotronix Healthcare and Servier, as a speaker or member of a speakers' bureau for Bayer AG, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, LivaNova, Meda Pharmaceuticals, Medtronic, MSD, Novartis Pharmaceuticals, Pfizer, Physiomed, St Jude Pharmaceuticals, and Servier.
Figures
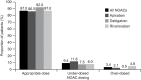
References
-
- Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(08):983–988. - PubMed
-
- ESC Scientific Document Group . Kirchhof P, Benussi S, Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. - PubMed
-
- Kirchhof P, Ammentorp B, Darius H. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF) Europace. 2014;16(01):6–14. - PMC - PubMed
-
- Ruff C T, Giugliano R P, Braunwald E.Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial Lancet 2015385(9984):2288–2295. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

