BMP antagonists in tissue development and disease
- PMID: 34435185
- PMCID: PMC8377005
- DOI: 10.1016/j.mbplus.2021.100071
BMP antagonists in tissue development and disease
Abstract
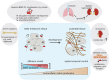
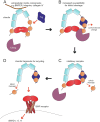
Bone morphogenic proteins (BMPs) are important growth regulators in embryogenesis and postnatal homeostasis. Their tight regulation is crucial for successful embryonic development as well as tissue homeostasis in the adult organism. BMP inhibition by natural extracellular biologic antagonists represents the most intensively studied mechanistic concept of BMP growth factor regulation. It was shown to be critical for numerous developmental programs, including germ layer specification and spatiotemporal gradients required for the establishment of the dorsal-ventral axis and organ formation. The importance of BMP antagonists for extracellular matrix homeostasis is illustrated by the numerous human connective tissue disorders caused by their mutational inactivation. Here, we will focus on the known functional interactions targeting BMP antagonists to the ECM and discuss how these interactions influence BMP antagonist activity. Moreover, we will provide an overview about the current concepts and investigated molecular mechanisms modulating BMP inhibitor function in the context of development and disease.
Keywords: ALK3, anaplastic lymphoma kinase 3; ATF2, activating transcription factor 2; ActR, activin receptor; BDB2, brachydactyly type B2; BISC, BMP-induced signalling complex; BMP antagonists; BMPER, BMP binding endothelial regulator; BMPs, bone morphogenetic proteins; Bone morphogenetic protein (BMP); CAN, cerberus and DAN; CDD, craniodiaphyseal dysplasia; CHRD domain, chordin specific domain; CUB domain, for complement C1r/C1s, Uegf, Bmp1 domain; Connective tissue disorder; Cv2, crossveinless-2; DAN, differential screening selected gene aberrative in neuroblastoma; DSD, diaphanospondylodysostosis; Dpp, decapentaplegic; ECM, extracellular matrix; ERK, extracellular signal-regulated kinases; Extracellular matrix (ECM); FMF, fibrillin microfibrils; HS, heparan sulphate; HSPGs, heparan sulphate proteoglycans; MAPKs, mitogen-activated protein kinases; MGC1, megalocornea 1; PI3K, phosphoinositide 3-kinase; PRDC, protein related to DAN and Cerberus; SOST, sclerostin; SYNS1, multiple synostoses syndrome 1; Scw, screw; Sog, short gastrulation; TCC, tarsal-carpal coalition syndrome; TGF-β, transforming growth factor- β; Tld, tolloid; Tsg, twisted gastrulation; VBCH, Van Buchem disease; Xlr/Tll, xolloid-related metalloprotease; vWC, von Willebrand factor type C; vWD, von Willebrand factor type D.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Wu M.Y., Hill C.S. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16(3):329–343. - PubMed
-
- Horbelt D., Denkis A., Knaus P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. Int. J. Biochem. Cell Biol. 2012;44(3):469–474. - PubMed
-
- Sieber C., Kopf J., Hiepen C., Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20(5–6):343–355. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

