Reductions in biomarkers of exposure to selected harmful and potentially harmful constituents following exclusive and partial switching from combustible cigarettes to myblu™ electronic nicotine delivery systems (ENDS)
- PMID: 34435305
- PMCID: PMC8964552
- DOI: 10.1007/s11739-021-02813-w
Reductions in biomarkers of exposure to selected harmful and potentially harmful constituents following exclusive and partial switching from combustible cigarettes to myblu™ electronic nicotine delivery systems (ENDS)
Abstract
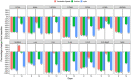
Electronic nicotine delivery systems (ENDS) offer adult combustible cigarette smokers an alternative, potentially reduced harm, mode of nicotine delivery, attributed to fewer and reduced levels of harmful and potentially harmful constituents (HPHCs) in their aerosols compared to cigarette smoke. These two identical, randomised, open label, two-part studies aimed to compare levels of 15 biomarkers of exposure (BoE) to selected HPHCs associated with tobacco smoking in healthy US adult smoker subjects (n = 72). Following 9 days of exclusive use of a range of allocated myblu™ ENDS variants, subjects' levels of 14 non-nicotine BoE were substantially reduced compared to baseline values (combustible cigarette use), in the range of 46-97%. BoE reductions were sustained in subjects who continued myblu use exclusively (n = 25) for a further 5 days, and returned to near baseline levels in subjects who returned to exclusive combustible cigarette use (n = 21). Dual users (n = 24) demonstrated reductions in BoE to a lesser extent than with exclusive myblu use. Measured nicotine equivalents did not significantly change throughout the study. These data suggest exclusive use of ENDS provides adult smokers seeking an alternative to combustible cigarettes with substantial reductions in HPHC exposures whilst achieving satisfying levels of nicotine delivery. Dual use involving substitution of cigarettes may also provide some of this advantage, but to lesser extent. Overall, the data contribute to the weight of evidence that ENDS are an important tool in tobacco harm reduction for adult smokers unwilling to or uninterested in quitting smoking. Study 1: NCT04430634, study 2: NCT04429932, clinicaltrials.gov (10-06-2020).
Keywords: Biomarkers of exposure; Cigarette; Electronic nicotine delivery systems; Smoking; Tobacco harm reduction; e-Cigarettes.
© 2021. The Author(s).
Conflict of interest statement
This work was funded by Fontem US LLC, a subsidiary of Imperial Brands PLC, and manufacturers of the
Figures


References
-
- Royal College of Physicians (2016) Nicotine without smoke: tobacco harm reduction. https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-toba.... Accessed 6 Aug 2021
-
- McNeill A, Brose LS, Calder R, Bauld L, Robson D (2018) Evidence review of ENDS and heated tobacco products 2018. A report commissioned by Public Health England, London
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

