Adult stem cell niches for tissue homeostasis
- PMID: 34435361
- PMCID: PMC9291197
- DOI: 10.1002/jcp.30562
Adult stem cell niches for tissue homeostasis
Abstract
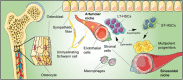
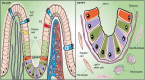
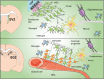
Adult stem cells are fundamental to maintain tissue homeostasis, growth, and regeneration. They reside in specialized environments called niches. Following activating signals, they proliferate and differentiate into functional cells that are able to preserve tissue physiology, either to guarantee normal turnover or to counteract tissue damage caused by injury or disease. Multiple interactions occur within the niche between stem cell-intrinsic factors, supporting cells, the extracellular matrix, and signaling pathways. Altogether, these interactions govern cell fate, preserving the stem cell pool, and regulating stem cell proliferation and differentiation. Based on their response to body needs, tissues can be largely classified into three main categories: tissues that even in normal conditions are characterized by an impressive turnover to replace rapidly exhausting cells (blood, epidermis, or intestinal epithelium); tissues that normally require only a basal cell replacement, though able to efficiently respond to increased tissue needs, injury, or disease (skeletal muscle); tissues that are equipped with less powerful stem cell niches, whose repairing ability is not able to overcome severe damage (heart or nervous tissue). The purpose of this review is to describe the main characteristics of stem cell niches in these different tissues, highlighting the various components influencing stem cell activity. Although much has been done, more work is needed to further increase our knowledge of niche interactions. This would be important not only to shed light on this fundamental chapter of human physiology but also to help the development of cell-based strategies for clinical therapeutic applications, especially when other approaches fail.
Keywords: bone marrow; central nervous system; heart; skeletal muscle; skin; stem cell niches; tissue homeostasis.
© 2021 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures


References
-
- Acar, M. , Kocherlakota, K. S. , Murphy, M. M. , Peyer, J. G. , Oguro, H. , Inra, C. N. , Jaiyeola, C. , Zhao, Z. , Luby‐Phelps, K. , & Morrison, S. J. (2015). Deep imaging of bone marrow shows non‐dividing stem cells are mainly perisinusoidal. Nature, 526(7571), 126–130. 10.1038/nature15250 - DOI - PMC - PubMed
-
- Andreotti, J. P. , Silva, W. N. , Costa, A. C. , Picoli, C. C. , Bitencourt, F. , Coimbra‐Campos, L. , Resende, R. R. , Magno, L. , Romano‐Silva, M. A. , Mintz, A. , & Birbrair, A. (2019). Neural stem cell niche heterogeneity. Seminars in Cell and Developmental Biology, 95, 42–53. 10.1016/j.semcdb.2019.01.005 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

