A phase 2b, randomized, double-blind, multicenter, vehicle-controlled study to assess the efficacy and safety of two crisaborole regimens in Japanese patients aged 2 years and older with mild-to-moderate atopic dermatitis
- PMID: 34435694
- PMCID: PMC9292399
- DOI: 10.1111/1346-8138.16120
A phase 2b, randomized, double-blind, multicenter, vehicle-controlled study to assess the efficacy and safety of two crisaborole regimens in Japanese patients aged 2 years and older with mild-to-moderate atopic dermatitis
Abstract
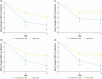
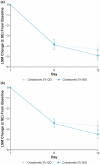
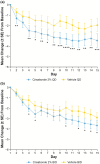
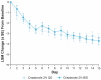
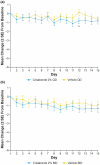
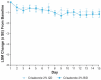
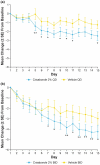
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritus, xerosis, and eczematous lesions. In Japan, treatment options, such as topical corticosteroids and tacrolimus, are associated with efficacy and safety concerns. Crisaborole ointment, 2%, is a topical non-steroidal anti-inflammatory agent approved in several countries for the treatment of mild-to-moderate AD. This phase 2b, randomized, double-blind study (NCT03954158) assessed the efficacy and safety of two crisaborole regimens versus vehicle in the treatment of Japanese patients aged ≥2 years with mild-to-moderate AD. Each patient was assigned to one of two age cohorts (≥12 or 2-11 years) and randomized to crisaborole once daily (QD) or twice daily (BID). All patients had two target lesions that were each randomly assigned to crisaborole or vehicle at baseline and treated for 2 weeks. The primary endpoint was change from baseline in total sign score (TSS) in crisaborole- or vehicle-treated target lesions on day 15, and secondary endpoints included change from baseline in Investigator's Static Global Assessment (ISGA) and pruritic assessments (Cohort 1: peak pruritus numeric rating scale [NRS]; Cohort 2: Itch Severity Scale Self-Report and Caregiver-Reported Itch Severity NRS) and incidence of treatment-emergent adverse events (TEAEs). This study comprised 81 patients (Cohort 1: n = 41; Cohort 2: n = 40). Crisaborole-treated lesions showed statistically significant reductions in TSS versus vehicle-treated lesions at day 15 (p < 0.01), and numerically larger decreases in TSS were observed with crisaborole BID versus crisaborole QD in both cohorts. Furthermore, crisaborole-treated lesions generally demonstrated greater decreases in ISGA, peak pruritus NRS, Itch Severity Scale, and Caregiver-Reported Itch Severity NRS versus vehicle-treated lesions irrespective of regimen or cohort. Overall, TEAEs were mild; the most frequently reported TEAEs was application site irritation. In summary, both crisaborole regimens, particularly crisaborole BID, demonstrated efficacy and were well tolerated.
Keywords: Japan; atopic dermatitis; clinical trial; pruritus; safety.
© 2021 Pfizer Inc. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
K. Fujita and M. Yoshida are employees of Pfizer R&D Japan, and K. Fujita is a stockholder of Pfizer Inc. M. Yagi has received research grants from Pfizer R&D Japan. S. Moriwaki has nothing to disclose. D. Graham is an employee and stockholder of Pfizer Inc.
Figures

References
-
- Boguniewicz M, Fonacier L, Guttman‐Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10–22.e12. - PubMed
-
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258.e1223. - PubMed
-
- Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol. 2021;32:23–33. - PubMed
-
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

