Brain Structure and Degeneration Staging in Friedreich Ataxia: Magnetic Resonance Imaging Volumetrics from the ENIGMA-Ataxia Working Group
- PMID: 34435700
- PMCID: PMC9292360
- DOI: 10.1002/ana.26200
Brain Structure and Degeneration Staging in Friedreich Ataxia: Magnetic Resonance Imaging Volumetrics from the ENIGMA-Ataxia Working Group
Abstract
Objective: Friedreich ataxia (FRDA) is an inherited neurological disease defined by progressive movement incoordination. We undertook a comprehensive characterization of the spatial profile and progressive evolution of structural brain abnormalities in people with FRDA.
Methods: A coordinated international analysis of regional brain volume using magnetic resonance imaging data charted the whole-brain profile, interindividual variability, and temporal staging of structural brain differences in 248 individuals with FRDA and 262 healthy controls.
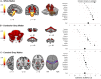
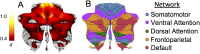
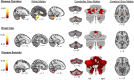
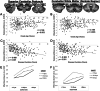
Results: The brainstem, dentate nucleus region, and superior and inferior cerebellar peduncles showed the greatest reductions in volume relative to controls (Cohen d = 1.5-2.6). Cerebellar gray matter alterations were most pronounced in lobules I-VI (d = 0.8), whereas cerebral differences occurred most prominently in precentral gyri (d = 0.6) and corticospinal tracts (d = 1.4). Earlier onset age predicted less volume in the motor cerebellum (rmax = 0.35) and peduncles (rmax = 0.36). Disease duration and severity correlated with volume deficits in the dentate nucleus region, brainstem, and superior/inferior cerebellar peduncles (rmax = -0.49); subgrouping showed these to be robust and early features of FRDA, and strong candidates for further biomarker validation. Cerebral white matter abnormalities, particularly in corticospinal pathways, emerge as intermediate disease features. Cerebellar and cerebral gray matter loss, principally targeting motor and sensory systems, preferentially manifests later in the disease course.
Interpretation: FRDA is defined by an evolving spatial profile of neuroanatomical changes beyond primary pathology in the cerebellum and spinal cord, in line with its progressive clinical course. The design, interpretation, and generalization of research studies and clinical trials must consider neuroanatomical staging and associated interindividual variability in brain measures. ANN NEUROL 2021;90:570-583.
© 2021 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures



References
-
- Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology 2014;42:174–183. - PubMed
-
- Delatycki MB, Bidichandani SI. Friedreich ataxia—pathogenesis and implications for therapies. Neurobiol Dis 2019;132:104606. - PubMed
-
- Reetz K, Dogan I, Costa AS, et al. Biological and clinical characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS) cohort: a cross‐sectional analysis of baseline data. Lancet Neurol 2015;14:174–182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

