Symptom Burden of Patients with Advanced Pancreas Cancer (APC): A Provincial Cancer Institute Observational Study
- PMID: 34436010
- PMCID: PMC8395517
- DOI: 10.3390/curroncol28040244
Symptom Burden of Patients with Advanced Pancreas Cancer (APC): A Provincial Cancer Institute Observational Study
Abstract
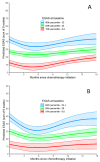
Patients with advanced pancreatic cancer (APC) experience many disease-related symptoms. ESAS-r measures the severity of 9 symptom domains and has been validated for use in the ambulatory oncology setting. We aimed to describe symptom burden at baseline for patients with APC treated with modern chemotherapy (CT), and to determine whether symptom burden at baseline is prognostic. Patients diagnosed with APC between 2012-2016, treated with ≥1 cycle of CT, who completed ≥1 ESAS-r were identified. Descriptive statistics were used to report symptom burden and common moderate-to-severe symptoms. A joint model was used to describe the trajectory of ESAS-r during follow-up while controlling for death. Multivariable Cox regression was used to identify independent predictors of death. Of 123 patients identified, the median age was 65 and 61% had metastatic disease. The median baseline ESAS-r total symptom distress score (TSDS) was 24. A total of 86% of patients had at least one symptom score of ≥4 at baseline, with the most common being: fatigue, nausea, anxiety, and shortness of breath. Median overall survival was 10.2 months. Baseline TSDS was not predictive for worse survival in the era of modern CT. Patients with APC have a high burden of cancer-associated symptoms and a high prevalence of moderate-to-severe symptoms. Early intervention has the potential to improve quality of life in this group of patients and should be investigated.
Keywords: ESAS; pancreatic cancer; patient-reported outcomes; prognosis; symptom burden.
Conflict of interest statement
C.A.K. has a research grant from Celgene Inc. The other authors declare no conflict of interest.
Figures


References
-
- SEER Cancer Stat Facts: Pancreatic Cancer. [(accessed on 12 November 2020)];2020 Available online: https://seer.cancer.gov/statfacts/html/pancreas.html.
-
- Torgerson S., Wiebe L.A. Supportive Care of the Patient with Advanced Pancreatic Cancer. Oncol. 2013;27:183–190. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

