Advancing Academic Cancer Clinical Trials Recruitment in Canada
- PMID: 34436014
- PMCID: PMC8395528
- DOI: 10.3390/curroncol28040248
Advancing Academic Cancer Clinical Trials Recruitment in Canada
Abstract
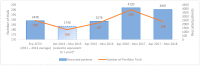
The Canadian Cancer Clinical Trials Network (3CTN) was established in 2014 to address the decline in academic cancer clinical trials (ACCT) activity. Funding was provided to cancer centres to conduct a Portfolio of ACCTs. Larger centres received core funding and were paired with smaller centres to enable support and sharing of resources. All centres were eligible for incentive-based funding for recruitment above pre-3CTN baseline. Established performance measures were collected and tracked. The overall recruitment target was 50% above pre-3CTN baseline by Year 4. An analysis was completed to identify predictive success factors and descriptive statistics were used to summarize site characteristics and outcomes. From 2014-2018, a total of 11,275 patients were recruited to 559 Portfolio trials, an overall increase of 59.6% above pre-3CTN baseline was observed in Year 4. Twenty-five (51%) adult centres met the Year 4 recruitment target and the overall recruitment target was met within three years. Three factors that correlated with sites' achieving recruitment targets were: time period, region and number of baseline trials. 3CTN was successful in meeting its objectives and will continue to support ACCTs and member cancer centres, monitor performance over time and seek continued funding to ensure success, better trial access and outcomes for patients.
Keywords: Canadian cancer clinical trials; academic cancer trials; funding; patient recruitment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Canadian Cancer Research Alliance . Report on the State of Cancer Clinical Trials in Canada. Canadian Cancer Research Alliance; Toronto, ON, Canada: 2011.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous