Wearable Biosensors for Non-Invasive Sweat Diagnostics
- PMID: 34436047
- PMCID: PMC8391966
- DOI: 10.3390/bios11080245
Wearable Biosensors for Non-Invasive Sweat Diagnostics
Abstract
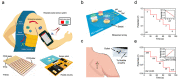
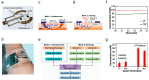
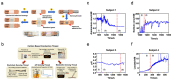
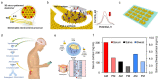
Recent advances in microfluidics, microelectronics, and electrochemical sensing methods have steered the way for the development of novel and potential wearable biosensors for healthcare monitoring. Wearable bioelectronics has received tremendous attention worldwide due to its great a potential for predictive medical modeling and allowing for personalized point-of-care-testing (POCT). They possess many appealing characteristics, for example, lightweight, flexibility, good stretchability, conformability, and low cost. These characteristics make wearable bioelectronics a promising platform for personalized devices. In this paper, we review recent progress in flexible and wearable sensors for non-invasive biomonitoring using sweat as the bio-fluid. Real-time and molecular-level monitoring of personal health states can be achieved with sweat-based or perspiration-based wearable biosensors. The suitability of sweat and its potential in healthcare monitoring, sweat extraction, and the challenges encountered in sweat-based analysis are summarized. The paper also discusses challenges that still hinder the full-fledged development of sweat-based wearables and presents the areas of future research.
Keywords: biomonitoring; biosensors; personalized healthcare; point-of-care; sweat.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Fang Y., Zhao X., Tat T., Xiao X., Chen G., Xu J., Chen J. All-in-One Conformal Epidermal Patch for Multimodal Biosensing. Matter. 2021;4:1102–1105. doi: 10.1016/j.matt.2021.03.005. - DOI

