Stem Cell Niche Microenvironment: Review
- PMID: 34436111
- PMCID: PMC8389324
- DOI: 10.3390/bioengineering8080108
Stem Cell Niche Microenvironment: Review
Abstract
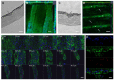
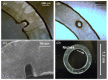
The cornea comprises a pool of self-regenerating epithelial cells that are crucial to preserving clarity and visibility. Limbal epithelial stem cells (LESCs), which live in a specialized stem cell niche (SCN), are crucial for the survival of the human corneal epithelium. They live at the bottom of the limbal crypts, in a physically enclosed microenvironment with a number of neighboring niche cells. Scientists also simplified features of these diverse microenvironments for more analysis in situ by designing and recreating features of different SCNs. Recent methods for regenerating the corneal epithelium after serious trauma, including burns and allergic assaults, focus mainly on regenerating the LESCs. Mesenchymal stem cells, which can transform into self-renewing and skeletal tissues, hold immense interest for tissue engineering and innovative medicinal exploration. This review summarizes all types of LESCs, identity and location of the human epithelial stem cells (HESCs), reconstruction of LSCN and artificial stem cells for self-renewal.
Keywords: cornea; microenvironment; niches; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

