Basidiobolus omanensis sp. nov. Causing Angioinvasive Abdominal Basidiobolomycosis
- PMID: 34436192
- PMCID: PMC8400364
- DOI: 10.3390/jof7080653
Basidiobolus omanensis sp. nov. Causing Angioinvasive Abdominal Basidiobolomycosis
Abstract
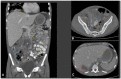
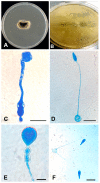
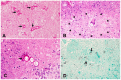
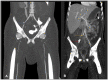
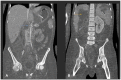
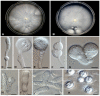
Human infectious fungal diseases are increasing, despite improved hygienic conditions. We present a case of gastrointestinal basidiobolomycosis (GIB) in a 20-year-old male with a history of progressively worsening abdominal pain. The causative agent was identified as a novel Basidiobolus species. Validation of its novelty was established by analysis of the partial ribosomal operon of two isolates from different organs. Phylogeny of ITS and LSU rRNA showed that these isolates belonged to the genus Basidiobolus, positioned closely to B. heterosporus and B. minor. Morphological and physiological data supported the identity of the species, which was named Basidiobolus omanensis, with CBS 146281 as the holotype. The strains showed high minimum inhibitory concentrations (MICs) to fluconazole (>64 µg/mL), itraconazole and voriconazole (>16 µg/mL), anidulafungin and micafungin (>16 µg/mL), but had a low MIC to amphotericin B (1 µg/mL). The pathogenic role of B. omanensis in gastrointestinal disease is discussed. We highlight the crucial role of molecular identification of these rarely encountered opportunistic fungi.
Keywords: Basidiobolus; ITS; LSU; basidiobolomycosis; gastrointestinal; morphology; phylogeny.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Van Overeem C. Uber ein merkwurdiges Vorkommen von Basidiobolus ranarum Eidam. Bull. Jardin Bot. 1925;7:423–431.
LinkOut - more resources
Full Text Sources

