Benzoic Acid and Its Hydroxylated Derivatives Suppress Early Blight of Tomato (Alternaria solani) via the Induction of Salicylic Acid Biosynthesis and Enzymatic and Nonenzymatic Antioxidant Defense Machinery
- PMID: 34436201
- PMCID: PMC8400885
- DOI: 10.3390/jof7080663
Benzoic Acid and Its Hydroxylated Derivatives Suppress Early Blight of Tomato (Alternaria solani) via the Induction of Salicylic Acid Biosynthesis and Enzymatic and Nonenzymatic Antioxidant Defense Machinery
Abstract
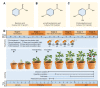
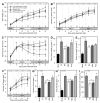
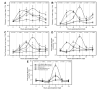
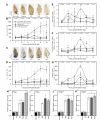
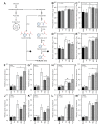
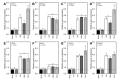
Tomato early blight, caused by Alternaria solani, is a destructive foliar fungal disease. Herein, the potential defensive roles of benzoic acid (BA) and two of its hydroxylated derivatives, ρ-hydroxybenzoic acid (HBA), and protocatechuic acid (PCA) against A. solani were investigated. All tested compounds showed strong dose-dependent fungistatic activity against A. solani and significantly reduced the disease development. Benzoic acid, and its hydroxylated derivatives, enhanced vegetative growth and yield traits. Moreover, BA and its derivatives induce the activation of enzymatic (POX, PPO, CAT, SlAPXs, and SlSODs) and non-enzymatic (phenolics, flavonoids, and carotenoids) antioxidant defense machinery to maintain reactive oxygen species (ROS) homeostasis within infected leaves. Additionally, BA and its hydroxylated derivatives induce the accumulation of salicylic acid (SA) and its biosynthetic genes including isochorismate synthase (SlICS), aldehyde oxidases (SlAO1 and SlAO2), and phenylalanine ammonia-lyases (SlPAL1, SlPAL2, SlPAL3, SlPAL5, and SlPAL6). Higher SA levels were associated with upregulation of pathogenesis-related proteins (SlPR-1, SlPR1a2, SlPRB1-2, SlPR4, SlPR5, SlPR6), nonexpressor of pathogenesis-related protein 1 (SlNPR1), and salicylic acid-binding protein (SlSABP2). These findings outline the potential application of BA and its hydroxylated derivatives as a sustainable alternative control strategy for early blight disease and also deciphering the physiological and biochemical mechanisms behind their protective role.
Keywords: Alternaria; antioxidant; benzoic acid; early blight; protocatechuic acid; reactive oxygen species (ROS); salicylic acid; tomato; ρ-hydroxybenzoic acid.
Conflict of interest statement
The authors declare that there is no conflict of interest and they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- FAOSTAT, Food and Agriculture Organization of the United Nations. [(accessed on 13 February 2020)]; Available online: http://www.fao.org/faostat/en/#data/QC.
-
- Abada K.A., Mostafa S.H., Mervat R., Abada K.A., Mostafa S.H., Mervat R. Effect of some chemical salts on suppressing the infection by early blight disease of tomato. Egypt. J. Appl. Sci. 2008;23:47–58.
-
- Raaijmakers J.M., Paulitz T.C., Steinberg C., Alabouvette C., Moënne-Loccoz Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 2009;321:341–361. doi: 10.1007/s11104-008-9568-6. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

