The Cardiac Neural Crest Cells in Heart Development and Congenital Heart Defects
- PMID: 34436231
- PMCID: PMC8397082
- DOI: 10.3390/jcdd8080089
The Cardiac Neural Crest Cells in Heart Development and Congenital Heart Defects
Abstract
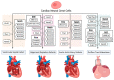
The neural crest (NC) is a multipotent and temporarily migratory cell population stemming from the dorsal neural tube during vertebrate embryogenesis. Cardiac neural crest cells (NCCs), a specified subpopulation of the NC, are vital for normal cardiovascular development, as they significantly contribute to the pharyngeal arch arteries, the developing cardiac outflow tract (OFT), cardiac valves, and interventricular septum. Various signaling pathways are shown to orchestrate the proper migration, compaction, and differentiation of cardiac NCCs during cardiovascular development. Any loss or dysregulation of signaling pathways in cardiac NCCs can lead to abnormal cardiovascular development during embryogenesis, resulting in abnormalities categorized as congenital heart defects (CHDs). This review focuses on the contributions of cardiac NCCs to cardiovascular formation, discusses cardiac defects caused by a disruption of various regulatory factors, and summarizes the role of multiple signaling pathways during embryonic development. A better understanding of the cardiac NC and its vast regulatory network will provide a deeper insight into the mechanisms of the associated abnormalities, leading to potential therapeutic advancements.
Keywords: cardiac neural crest; congenital heart defects (CHDs); heart development; neural crest cells (NCCs); outflow tract (OFT).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Xi M., Lui F. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2021. Neuroanatomy, Neural Crest. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

