Detection of Prostate Cancer via IR Spectroscopic Analysis of Urinary Extracellular Vesicles: A Pilot Study
- PMID: 34436354
- PMCID: PMC8401611
- DOI: 10.3390/membranes11080591
Detection of Prostate Cancer via IR Spectroscopic Analysis of Urinary Extracellular Vesicles: A Pilot Study
Abstract
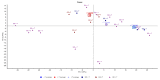
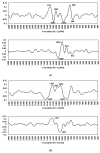
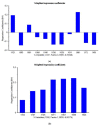
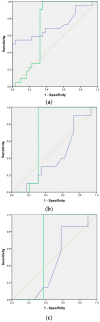
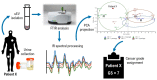
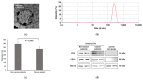
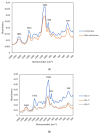
Extracellular vesicles (EVs) are membranous nanoparticles naturally released from living cells which can be found in all types of body fluids. Recent studies found that cancer cells secreted EVs containing the unique set of biomolecules, which give rise to a distinctive absorbance spectrum representing its cancer type. In this study, we aimed to detect the medium EVs (200-300 nm) from the urine of prostate cancer patients using Fourier transform infrared (FTIR) spectroscopy and determine their association with cancer progression. EVs extracted from 53 urine samples from patients suspected of prostate cancer were analyzed and their FTIR spectra were preprocessed for analysis. Characterization of morphology, particle size and marker proteins confirmed that EVs were successfully isolated from urine samples. Principal component analysis (PCA) of the EV's spectra showed the model could discriminate prostate cancer with a sensitivity of 59% and a specificity of 81%. The area under curve (AUC) of FTIR PCA model for prostate cancer detection in the cases with 4-20 ng/mL PSA was 0.7, while the AUC for PSA alone was 0.437, suggesting the analysis of urinary EVs described in this study may offer a novel strategy for the development of a noninvasive additional test for prostate cancer screening.
Keywords: EVs; FTIR; diagnosis; exosomes; prostate cancer; urine test.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- GLOBOCAN 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. [(accessed on 31 July 2021)]; Available online: http://globocan.iarc.fr/Default.aspx.
-
- Chou R., Croswell J.M., Dana T., Bougatsos C., Blazina I., Fu R., Gleitsmann K., Koenig H.C., Lam C., Maltz A., et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

