Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids
- PMID: 34436444
- PMCID: PMC8399526
- DOI: 10.3390/metabo11080503
Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids
Abstract
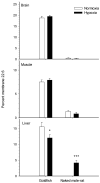
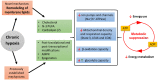
Metabolic suppression is an essential strategy to cope with chronic hypoxia. This review examines the physiological processes used to survive in low oxygen environments. It proposes a novel mechanism-the remodeling of membrane lipids-to suppress ATP use and production. Temperature (homeoviscous adaptation), diet (natural doping in migrant birds) and body mass (membrane pacemaker of metabolism) have an impact on the lipid composition of membranes, which, in turn, modulates metabolic capacity. Vertebrate champions of hypoxia tolerance show extensive changes in membrane lipids upon in vivo exposure to low oxygen. These changes and those observed in hibernating mammals can promote the downregulation of ion pumps (major ATP consumers), ion channels, mitochondrial respiration capacity (state 3, proton leak, cytochrome c oxidase), and energy metabolism (β-oxidation and glycolysis). A common membrane signal regulating the joint inhibition of ion pumps and channels could be an exquisite way to preserve the balance between ATP supply and demand in hypometabolic states. Membrane remodeling together with more traditional mechanisms could work in concert to cause metabolic suppression.
Keywords: Na+/K+-ATPase; cholesterol; energy metabolism; fatty acids; hypometabolism; hypoxia tolerance; low oxygen stress; membrane remodeling; metabolic suppression; mitochondrial respiration; phospholipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Richards J.G., Farrell A.P., Brauner C.J. Hypoxia. 1st ed. Volume 27. Academic Press; London, UK: 2009. p. 528.
-
- Lutz P.L., Storey K.B. Comprehensive Physiology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2010. Adaptations to variations in oxygen tension by vertebrates and invertebrates; pp. 1479–1522.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

