Secretion of Pertussis Toxin from Bordetella pertussis
- PMID: 34437445
- PMCID: PMC8402538
- DOI: 10.3390/toxins13080574
Secretion of Pertussis Toxin from Bordetella pertussis
Abstract
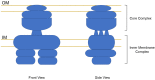
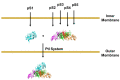
Production and secretion of pertussis toxin (PT) is essential for the virulence of Bordetella pertussis. Due to the large oligomeric structure of PT, transport of the toxin across bacterial membrane barriers represents a significant hurdle that the bacteria must overcome in order to maintain pathogenicity. During the secretion process, PT undergoes a two-step transport process. The first step involves transport of the individual polypeptide chains of PT across the inner membrane utilizing a generalized secretion pathway, most likely the bacterial Sec system. The second step involves the use of a specialized apparatus to transport the toxin across the outer membrane of the bacterial cell. This apparatus, which has been termed the Ptl transporter and which is unique to the PT secretion pathway, is a member of the type IV family of bacterial transporters. Here, the current understanding of the PT secretion process is reviewed including a description of the Ptl proteins that assemble to form the transporter, the general structure of type IV transporters, the known similarities and differences between canonical type IV substrate transport and Ptl-mediated transport of PT, as well as the known sequence of events in the assembly and secretion of PT.
Keywords: pertussis toxin; toxin secretion; type IV secretion.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Nicosia A., Perugini M., Franzini C., Casagli M.C., Borri M.G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: Operon structure and gene duplication. Proc. Natl. Acad. Sci. USA. 1986;83:4631–4635. doi: 10.1073/pnas.83.13.4631. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

