SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status - Los Angeles County, California, May 1-July 25, 2021
- PMID: 34437525
- PMCID: PMC8389389
- DOI: 10.15585/mmwr.mm7034e5
SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status - Los Angeles County, California, May 1-July 25, 2021
Abstract
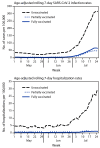
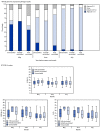
COVID-19 vaccines fully approved or currently authorized for use through Emergency Use Authorization from the Food and Drug Administration are critical tools for controlling the COVID-19 pandemic; however, even with highly effective vaccines, a proportion of fully vaccinated persons will become infected with SARS-CoV-2, the virus that causes COVID-19 (1). To characterize postvaccination infections, the Los Angeles County Department of Public Health (LACDPH) used COVID-19 surveillance and California Immunization Registry 2 (CAIR2) data to describe age-adjusted infection and hospitalization rates during May 1-July 25, 2021, by vaccination status. Whole genome sequencing (WGS)-based SARS-CoV-2 lineages and cycle threshold (Ct) values from qualitative reverse transcription-polymerase chain reaction (RT-PCR) for two SARS-CoV-2 gene targets, including the nucleocapsid (N) protein gene region and the open reading frame 1 ab (ORF1ab) polyprotein gene region,* were reported for a convenience sample of specimens. Among 43,127 reported SARS-CoV-2 infections in Los Angeles County residents aged ≥16 years, 10,895 (25.3%) were in fully vaccinated persons, 1,431 (3.3%) were in partially vaccinated persons, and 30,801 (71.4%) were in unvaccinated persons. Much lower percentages of fully vaccinated persons infected with SARS-CoV-2 were hospitalized (3.2%), were admitted to an intensive care unit (0.5%), and required mechanical ventilation (0.2%) compared with partially vaccinated persons (6.2%, 1.0%, and 0.3%, respectively) and unvaccinated persons (7.6%, 1.5%, and 0.5%, respectively) (p<0.001 for all comparisons). On July 25, the SARS-CoV-2 infection rate among unvaccinated persons was 4.9 times and the hospitalization rate was 29.2 times the rates among fully vaccinated persons. During May 1-July 25, the percentages of B.1.617.2 (Delta) variant infections estimated from 6,752 samples with lineage data increased among fully vaccinated persons (from 8.6% to 91.2%), partially vaccinated persons (from 0% to 88.1%), and unvaccinated persons (from 8.2% to 87.1%). In May, there were differences in median Ct values by vaccination status; however, by July, no differences were detected among specimens from fully vaccinated, partially vaccinated, and unvaccinated persons by gene targets. These infection and hospitalization rate data indicate that authorized vaccines were protective against SARS-CoV-2 infection and severe COVID-19 during a period when transmission of the Delta variant was increasing. Efforts to increase COVID-19 vaccination, in coordination with other prevention strategies, are critical to preventing COVID-19-related hospitalizations and deaths.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. M. Claire Jarashow reports consulting fees from Uber outside the current work and unpaid board membership of two international nongovernmental organizations (C2C and Developing Communities) outside the current work. No other potential conflicts of interest were disclosed.
Figures
References
-
- Allen H, Vusirikala A, Flannagan J, et al.; Public Health England. Increased household transmission of COVID-19 cases associated with SARS-CoV-2 variant of concern B.1.617.2: a national case-control study. Knowledge Hub [Preprint posted online June 18, 2021]. https://khub.net/documents/135939561/405676950/Increased+Household+Trans...
-
- Nasreen S, He S, Chung H, et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada [Preprint posted online July 16, 2021]. https://www.medrxiv.org/content/10.1101/2021.06.28.21259420v2 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

