Catalytic Enantioselective Access to Dihydroquinoxalinones via Formal α-Halo Acyl Halide Synthon in One Pot
- PMID: 34437760
- PMCID: PMC8596509
- DOI: 10.1002/anie.202110173
Catalytic Enantioselective Access to Dihydroquinoxalinones via Formal α-Halo Acyl Halide Synthon in One Pot
Abstract
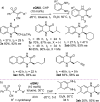
An enantioselective one-pot catalytic strategy to dihydroquinoxalinones, featuring novel 1-phenylsulfonyl-1-cyano enantioenriched epoxides as masked α-halo acyl halide synthons, followed by a domino ring-opening cyclization (DROC), is documented. A popular quinine-derived urea served as the catalyst in two out of the three steps performed in the same solvent using commercially available aldehydes, (phenylsulfonyl)acetonitrile, cumyl hydroperoxide and 1,2-phenylendiamines. Medicinally relevant 3-aryl/alkyl-substituted heterocycles are isolated in generally good to high overall yield and high enantioselectivity (up to 99 % ee). A rare example of excellent reusability of an organocatalyst at higher scale, subjected to oxidative conditions, is demonstrated. Mechanistically, labile α-ketosulfone has been detected as the intermediate involved in the DROC process. Theoretical calculations on the key epoxidation step rationalize the observed stereocontrol, highlighting the important role played by the sulfone group.
Keywords: asymmetric catalysis; domino ring-opening cyclization; epoxidation; heterocycles; one-pot reactions.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

