JAK inhibitors in the treatment of atopic dermatitis
- PMID: 34437922
- PMCID: PMC10166130
- DOI: 10.1016/j.jaci.2021.08.009
JAK inhibitors in the treatment of atopic dermatitis
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disorder associated with heterogenous presentation and often immense patient burden. Safe, targeted treatment options are currently limited. This focused review of the published literature, including clinical trial results, case reports, and abstracts, as well as presentations from scientific meetings and data from industry press releases, describes the use of topical and systemic Janus kinase (JAK) inhibitors in the treatment of AD. New topical JAK inhibitors include ruxolitinib (JAK1/2) and delgocitinib (pan-JAK). Ruxolitinib cream met all primary and secondary endpoints in phase 3 clinical trials for mild-to-moderate AD with minimal treatment-emergent adverse events. Delgocitinib ointment was recently approved in Japan for pediatric and adult AD. Oral JAK inhibitors include baricitinib (JAK1/2), abrocitinib (JAK1-selective), and upadacitinib (JAK1-selective). All 3 met primary and secondary endpoints across numerous trials for moderate-to-severe AD. Treatment-emergent adverse events were mainly mild to moderate and included acne, nausea, headache, upper respiratory tract infection, and to a lesser degree, herpes infection and selected laboratory abnormalities. JAK inhibitors hold great promise as the next generation of targeted AD therapy. While their outstanding efficacy is balanced by a favorable safety profile in clinical trials, real-world data are needed to better understand long-term safety, durability, and treatment success.
Keywords: Atopic dermatitis; JAK inhibitor; eczema; itch; therapy.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: Raj Chovatiya reports personal fees from Abbvie, Incyte, Regeneron, and Sanofi-Genzyme. Amy S. Paller reports personal fees from AbbVie, Arena, Bausch, Bristol Myer Squibb, Dermavant, Eli Lilly, Forte, Leo, Lifemax, Novartis, Pfizer, RAPT, Regeneron, and Sanofi. She is an investigator (funding to institution) for AbbVie, Anaptysbio, Eli Lilly, Incyte, Janssen, Regeneron, and UCB.
Figures
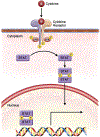

References
-
- Hua T, Silverberg JI. Atopic dermatitis in US adults: Epidemiology, association with marital status, and atopy. Ann Allergy Asthma Immunol 2018; 121:622–4. - PubMed
-
- Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Health utility scores of atopic dermatitis in US adults. J Allergy Clin Immunol Pract 2019; 7:1246–52 e1. - PubMed
-
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol 2019; 80:390–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

