Release of chromatin extracellular traps by phagocytes of Atlantic salmon, Salmo salar (Linnaeus, 1758)
- PMID: 34438058
- PMCID: PMC8653909
- DOI: 10.1016/j.fsi.2021.08.023
Release of chromatin extracellular traps by phagocytes of Atlantic salmon, Salmo salar (Linnaeus, 1758)
Abstract
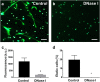
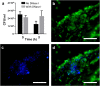
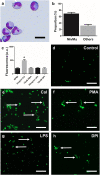
Neutrophils release chromatin extracellular traps (ETs) as part of the fish innate immune response to counter the threats posed by microbial pathogens. However, relatively little attention has been paid to this phenomenon in many commercially farmed species, despite the importance of understanding host-pathogen interactions and the potential to influence ET release to reduce disease outbreaks. The aim of this present study was to investigate the release of ETs by Atlantic salmon (Salmo salar L.) immune cells. Extracellular structures resembling ETs of different morphology were observed by fluorescence microscopy in neutrophil suspensions in vitro, as these structures stained positively with Sytox Green and were digestible with DNase I. Immunofluorescence studies confirmed the ET structures to be decorated with histones H1 and H2A and neutrophil elastase, which are characteristic for ETs in mammals and other organisms. Although the ETs were released spontaneously, release in neutrophil suspensions was stimulated most significantly with 5 μg/ml calcium ionophore (CaI) for 1 h, whilst the fish pathogenic bacterium Aeromonas salmonicida (isolates 30411 and Hooke) also exerted a stimulatory effect. Microscopic observations revealed bacteria in association with ETs, and fewer bacterial colonies of A. salmonicida Hooke were recovered at 3 h after co-incubation with neutrophils that had been induced to release ETs. Interestingly, spontaneous release of ETs was inversely associated with fish mass (p < 0.05), a surrogate for age. Moreover, suspensions enriched for macrophages and stimulated with 5 μg/ml CaI released ET-like structures that occasionally led to the formation of large clumps of cells. A deeper understanding for the roles and functions of ETs within innate immunity of fish hosts, and their interaction with microbial pathogens, may open new avenues towards protecting cultured stocks against infectious diseases.
Keywords: ETosis; Macrophage; NETosis; Neutrophil extracellular traps; Polymorphonucleocyte.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors confirm they have no known conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

