Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1
- PMID: 34438069
- PMCID: PMC8382485
- DOI: 10.1016/j.cmi.2021.07.031
Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1
Abstract
Objectives: The immunogenicity and safety of the Pfizer-BioNTech BNT162b2 mRNA vaccine in people living with human immunodeficiency virus type 1 (PLWH) are unknown. We aimed to assess the immunogenicity and safety of this vaccine in PLWH.
Methods: In this prospective open study, we enrolled 143 PLWH, aged ≥18 years, who attended our clinic and 261 immunocompetent health-care workers (HCWs). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor binding domain (RBD) IgG and neutralizing antibodies were measured. Adverse events, viral load and CD4 cell counts were monitored.
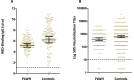
Results: At a median of 18 days (interquartile range 14-21 days) after the second dose, anti-RBD-IgG was positive in 139/141 (98%) PLWH. Among HCWs, 258/261 (98.9%) developed anti-RBD-IgG at a median of 26 days (interquartile range 24-27 days) after the second dose. Following the second dose, immune sera neutralized SARS-CoV-2 pseudo-virus in 97% and 98% of PLWH and HCWs, respectively. Adverse events were reported in 60% of PLWH, mainly pain at the injection site, fatigue and headache. AIDS-related adverse events were not reported. Human immunodeficiency virus load increased in 3/143 (2%) patients from <40 copies/mL to ≤100 copies/mL. CD4+ T-cell count decreased from a geometric mean of 700 cells/μL (95% CI 648-757 cells/μL) to 633.8 cells/μL (95% CI 588-683 cells/μL) (p < 0.01).
Conclusions: BNT162b2 mRNA vaccine appears immunogenic and safe in PLWH who are on antiretroviral therapy with unsuppressed CD4 count and suppressed viral load.
Keywords: BNT162b2; Coronavirus disease 2019; Human immunodeficiency virus; Immunogenicity; Vaccination.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Tasker S.A., O’Brien W.A., Treanor J.J., Weiss P.J., Olson P.E., Kaplan A.H., et al. Effects of influenza vaccination in HIV-infected adults: a double-blind, placebo controlled trial. Vaccine. 1998;16:1039–1042. - PubMed
-
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M, Becker M, Slater D, et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020 preprint.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

