Numerical simulations for sonochemistry
- PMID: 34438317
- PMCID: PMC8387904
- DOI: 10.1016/j.ultsonch.2021.105728
Numerical simulations for sonochemistry
Abstract
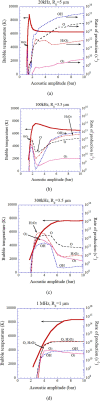
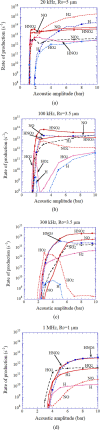
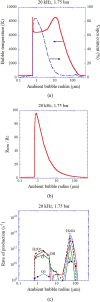
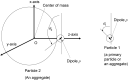
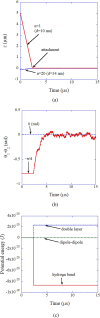
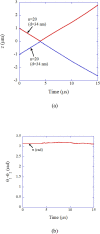
Numerical simulations for sonochemistry are reviewed including single-bubble sonochemistry, influence of ultrasonic frequency and bubble size, acoustic field, and sonochemical synthesis of nanoparticles. The theoretical model of bubble dynamics including the effect of non-equilibrium chemical reactions inside a bubble has been validated from the study of single-bubble sonochemistry. By the numerical simulations, it has been clarified that there is an optimum bubble temperature for the production of oxidants inside an air bubble such as OH radicals and H2O2 because at higher temperature oxidants are strongly consumed inside a bubble by oxidizing nitrogen. Unsolved problems are also discussed.
Keywords: Bubble dynamics model; Numerical simulation; OH radical; Optimum bubble temperature; Single-bubble sonochemistry; Sonochemical synthesis of nanoparticles.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
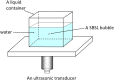

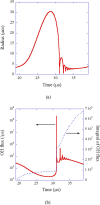
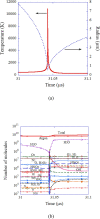
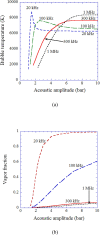




References
-
- H. Nomura, S. Koda, What is sonochemistry?, in: F.Grieser, P.Choi, N. Enomoto, H. Harada, K. Okitsu, K. Yasui (Eds.), Sonochemistry and the Acoustic Bubble, Elsevier, Amsterdam, 2015, pp. 1-9, 10.1016/B978-0-12-801530-8.00001-3.
-
- D.F. Gaitan, L.A. Crum, Observation of sonoluminescence from a single, stable cavitation bubble in a water glycerin mixture, in: M.F. Hamilton, D.T.Blackstock (Eds.), Frontiers of Nonlinear Acoustics, Proc. 12th ISNA, Elsevier Appl. Sci. Pub., 1990, pp. 459-463.
-
- Yasui K., Tuziuti T., Sivakumar M., Iida Y. Sonoluminescence. Appl. Spectrosc. Rev. 2004;39(3):399–436. doi: 10.1081/ASR-200030202. - DOI
-
- Young F.R. CRC Press; Boca Raton: 2005. Sonoluminescence.
Publication types
LinkOut - more resources
Full Text Sources

