Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic
- PMID: 34438338
- PMCID: PMC8363177
- DOI: 10.1016/j.bmc.2021.116366
Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic
Abstract
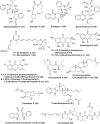
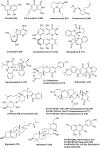
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, which started in late 2019, drove the scientific community to conduct innovative research to contain the spread of the pandemic and to care for those already affected. Since then, the search for new drugs that are effective against the virus has been strengthened. Featuring a relatively low cost of production under well-defined methods of cultivation, fungi have been providing a diversity of antiviral metabolites with unprecedented chemical structures. In this review, we present viral RNA infections highlighting SARS-CoV-2 morphogenesis and the infectious cycle, the targets of known antiviral drugs, and current developments in this area such as drug repurposing. We also explored the metabolic adaptability of fungi during fermentation to produce metabolites active against RNA viruses, along with their chemical structures, and mechanisms of action. Finally, the state of the art of research on SARS-CoV-2 inhibitors of fungal origin is reported, highlighting the metabolites selected by docking studies.
Keywords: Antiviral compounds; Drug discovery; Fungi secondary metabolites; Molecular docking; SARS-CoV-2.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- WHO - World Health Organization. Prioritizing diseases for research and development in emergency contexts; 2020. https://www.who.int/activities/prioritizing-diseases-for-research-and-de.... Accessed 3 November 2020.
-
- WHO - World Health Organization. WHO Coronavirus (COVID-19) Dashboard; 2021. https://covid19.who.int. Accessed 6 July 2021.
-
- WHO - World Health Organization. COVID-19 vaccine tracker and landscape; 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... Accessed 6 July 2021.
-
- Craven J. Covid-19 Vaccine Tracker. Regulatory Affairs Professionals Society; 2021. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vac.... Accessed 6 July 2021.
-
- WHO - World Health Organization. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process; 2021. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVI.... Accessed 6 July 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

