Colchicine for acute gout
- PMID: 34438469
- PMCID: PMC8407279
- DOI: 10.1002/14651858.CD006190.pub3
Colchicine for acute gout
Abstract
Background: This is an updated Cochrane Review, first published in 2006 and updated in 2014. Gout is one of the most common rheumatic diseases worldwide. Despite the use of colchicine as one of the first-line therapies for the treatment of acute gout, evidence for its benefits and harms is relatively limited.
Objectives: To update the available evidence of the benefits and harms of colchicine for the treatment of acute gout.
Search methods: We updated the search of CENTRAL, MEDLINE, Embase, Clinicaltrials.gov and WHO ICTRP registries to 28 August 2020. We did not impose any date or language restrictions in the search.
Selection criteria: We considered published randomised controlled trials (RCTs) and quasi-randomised controlled trials (quasi-RCTs) evaluating colchicine therapy compared with another therapy (placebo or active) in acute gout; low-dose colchicine at clinically relevant doses compared with placebo was the primary comparison. The major outcomes were pain, participant global assessment of treatment success (proportion with 50% or greater decrease in pain from baseline up to 32 to 36 hours), reduction of inflammation, function of target joint, serious adverse events, total adverse events and withdrawals due to adverse events.
Data collection and analysis: We used standard methodological procedures as expected by Cochrane in this review update.
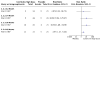
Main results: We included four trials (803 randomised participants), including two new trials, in this updated review. One three-arm trial compared high-dose colchicine (52 participants), low-dose colchicine (74 participants) and placebo (59 participants); one trial compared high-dose colchicine with placebo (43 participants); one trial compared low-dose colchicine with non-steroidal anti-inflammatory drugs (NSAIDs) (399 participants); and one trial compared low-dose colchicine with Chuanhu anti-gout mixture (traditional Chinese Medicine compound) (176 participants). We did not identify any trials comparing colchicine to glucocorticoids (by any route). The mean age of participants ranged from 51.2 to 70 years, and trial duration from 48 hours to 12 weeks. Two trials were at low risk of bias, one was possibly susceptible to selection bias (random sequence generation), reporting bias and other bias, and one open-label trial was at high risk of performance and detection bias. For the primary comparison, low-quality evidence from one trial (103 participants, downgraded for imprecision and bias) suggests low-dose colchicine may improve treatment outcome compared to placebo with little or no increased risk of adverse events. The number of people who reported treatment success (50% or greater pain reduction) at 32 to 36 hours was slightly larger with low-dose colchicine (418 per 1000) compared with placebo (172 per 1000; risk ratio (RR) 2.43, 95% confidence interval (CI) 1.05 to 5.64; absolute improvement 25% more reported success (7% more to 42% more, the 95% CIs include both a clinically important and unimportant benefit); relative change of 143% more people reported treatment success (5% more to 464% more). The incidence of total adverse events was 364 per 1000 with low-dose colchicine compared with 276 per 1000 with placebo: RR 1.32, 95% CI 0.68 to 2.56; absolute difference 9% more events with low-dose colchicine (9% fewer to 43% more, the 95% CIs include both a clinically important effect and no effect); relative change of 32% more events (32% fewer to 156% more). No participants withdrew due to adverse events or reported any serious adverse events. Pain, inflammation and function were not reported. Low-quality evidence (downgraded for imprecision and bias) from two trials (124 participants) suggests that high-dose colchicine compared to placebo may improve symptoms, but with increased risk of harms. More participants reported treatment success at 32 to 36 hours with high-dose colchicine (518 per 1000) compared with placebo (240 per 1000): RR 2.16, 95% CI 1.28 to 3.65, absolute improvement 28% (8% more to 46% more); more also had reduced inflammation at this time point with high-dose colchicine (504 per 1000) compared with placebo (48 per 1000): RR 10.50, 95% CI 1.48 to 74.38; absolute improvement 45% greater (22% greater to 68% greater); but more adverse events were reported with high-dose colchicine (829 per 1000 compared with 260 per 1000): RR 3.21, 95% CI 2.01 to 5.11, absolute difference 57% (26% more to 74% more). Pain and function were not reported. Low-quality evidence from a single trial comparing high-dose to low-dose colchicine indicates there may be little or no difference in benefit in terms of treatment success at 32 to 36 hours but more adverse events associated with the higher dose. Similarly, low-quality evidence from a single trial indicates there may also be little or no benefit of low-dose colchicine over NSAIDs in terms of treatment success and pain reduction at seven days, with a similar number of adverse events reported at four weeks follow-up. Reduction of inflammation, function of target joint and withdrawals due to adverse events were not reported in either of these trials, and pain was not reported in the high-dose versus low-dose colchicine trial. We were unable to estimate the risk of serious adverse events for most comparisons as there were few events reported in the trials. One trial (399 participants) reported three serious adverse (one in a participant receiving low-dose colchicine and two in participants receiving NSAIDs), due to reasons unrelated to the trial (low-quality evidence downgraded for bias and imprecision).
Authors' conclusions: We found low-quality evidence that low-dose colchicine may be an effective treatment for acute gout when compared to placebo and low-quality evidence that its benefits may be similar to NSAIDs. We downgraded the evidence for bias and imprecision. While both high- and low-dose colchicine improve pain when compared to placebo, low-quality evidence suggests that high-dose (but not low-dose) colchicine may increase the number of adverse events compared to placebo, while low-quality evidence indicates that the number of adverse events may be similar with low-dose colchicine and NSAIDs. Further trials comparing colchicine to placebo or other treatment will likely have an important impact on our confidence in the effect estimates and may change the conclusions of this review. There are no trials reporting the effect of colchicine in populations with comorbidities or in comparison with other commonly used treatments, such as glucocorticoids.
Trial registration: ClinicalTrials.gov NCT01994226.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Bayden J McKenzie has no declarations of interest.
Mihir D Wechalekar has no declaration of interest for this review; he has received research grants for unrelated work in a different disease area from Janssen Research (Philadelphia, USA).
Renea V Johnston is the Managing Editor of Cochrane Musculoskeletal, but was not involved in editorial decisions regarding this review. She is a recipient of a NHMRC (Australia) Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funders do not participate in the conduct of this review. RJ has no declarations of interest.
Naomi Schlesinger has no declaration of interest for this review; she has a research grant from AMGEN and has received honorarium from Horizon pharmaceutics.
Rachelle Buchbinder is the Co‐ordinating Editor of Cochrane Musculoskeletal, but was not involved in editorial decisions regarding this review. She is a recipient of a National Health and Medical Research Council (NHMRC) Cochrane Collaboration Round 7 Funding Program Grant, which supports the activities of Cochrane Musculoskeletal Australia and Cochrane Australia, but the funders do not participate in the conduct of reviews. She has no declarations of interest.
Figures






















Update of
-
Colchicine for acute gout.Cochrane Database Syst Rev. 2014 Aug 15;(8):CD006190. doi: 10.1002/14651858.CD006190.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2021 Aug 26;8:CD006190. doi: 10.1002/14651858.CD006190.pub3. PMID: 25123076 Updated.
Comment in
-
How safe and effective is the use of colchicine for the treatment of gout flares? A Cochrane Review summary with commentary.Int J Rheum Dis. 2022 May;25(5):613-616. doi: 10.1111/1756-185X.14318. Epub 2022 Mar 21. Int J Rheum Dis. 2022. PMID: 35312169 No abstract available.
References
References to studies included in this review
Ahern 1987 {published data only}
-
- Ahern MJ, Reid C, Gordon TP. Does colchicine work? Results of the first controlled study in gout. Australian and New Zealand Journal of Medicine 1987;17:301-4. - PubMed
Roddy 2020 {published data only}69836939
Terkeltaub 2010 {published data only}
-
- Furst D, Maranian E, Davis P, Wason MW, Khanna S, Terkeltaub D. Chronologic age, renal function, and comorbid conditions (physiologic age) of patients with gout did not increase likelihood of adverse events (AEs): AGREE study post hoc analyses. Arthritis and Rheumatism 2009;62(Suppl 10):147.
-
- Terkeltaub R, Furst D, Bennett E, Kook K, Crockett K, Davis RS, et al. Colchicine efficacy assessed by time to 50 reduction of pain is comparable in low dose and high dose regimens: secondary analyses of the agree trial. Arthritis and Rheumatism 2010;60(Suppl 10):1103.
-
- Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomised, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis and Rheumatism 2010;62(4):1060-8. - PubMed
-
- Wason S, Lauterio T, Crockett S, Davis, M. Colchicine, as assessed by target joint pain scores, is effective at 16 hours in patients with acute gout flares. Arthritis and Rheumatism 2012;64(Suppl 10):S812.
References to studies excluded from this review
Blank 2010 {published data only}
-
- Blank N. Acute gouty arthropathy: treatment of acute gout attack with colchicine. Medizinische Klinik 2010;105(9):669-70.
ChiCTR1800020315 {published data only}
-
- ChiCTR1800020315. Study for the effectiveness of different courses of low-dose colchicine for preventing acute flares during urate lowering therapy by febuxostat. www.chictr.org.cn/showproj.aspx?proj=32164 (first received 23 December 2018).
Hill 2013 {published data only}
-
- Hill E, Higgs JB, Sky K, Sit M, Collamer AN. Does starting allopurinol prolong acute treated gout? Arthritis and Rheumatism 2013;65(Suppl 10):S730. - PubMed
Karimzadeh 2006 {published data only}
-
- Karimzadeh H, Nazari J, Mottaghi P, Kabiri P. Different duration of colchicine for preventing recurrence of gouty arthritis. Journal of Research in Medical Sciences 2006;11(2):104-7.
Liu 2015 {published data only}
-
- Liu Y, Li Z-C, Chen J-B, Yu T-T, Cui C, Zhang H-B. Therapeutic efficacy of small doses of colchicine combined with glucocorticoid for acute gouty arthritis. People's Military Medical Press 2015;40(8):652.
Moon 2011 {published data only}
-
- Moon KT. Low-dose colchicine effective for acute gout flare-ups. American Family Physician 2011;83(3):316.
Paulus 1974 {published data only}
-
- Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo controlled study of probenecid treated patients. Arthritis and Rheumatism 1974;17(5):609-14. - PubMed
Rapado 1975 {published data only}
-
- Rapado A. Influence of atmospheric pressure on acute attacks of gout: negative findings. Revista Espanola de Reumatologia 1975;2(4):188-93.
Schlesinger 2002 {published data only}
-
- Schlesinger N, Baker DG, Beutler AM, Hoffman BI, Schumacher Hr Jr. Local ice therapy during bouts of acute gouty arthritis. Journal of Rheumatology 2002;29(2):331-4. - PubMed
Schlesinger 2011 {published data only}
-
- Schlesinger N, Lin H-Y, De Meulemeester M, Nasonov E, Rovensky J, Arulmani U, et al. Efficacy of canakinumab (ACZ885) in the prevention of flares in gout patients initiating allopurinol therapy. NDT Plus 2010;3:iii3-4.
Yamanaka 2018 {published data only}
-
- Yamanaka H, Tamaki S, Ide Y, Kim H, Inoue K, Sugimoto M. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Annals of the Rheumatic Diseases 2018;77(2):270-6. - PMC - PubMed
Yang 2018 {published data only}
-
- Yang D-H, Chen H-C, Wei JC-C. Early lowering of serum uric acid levels in the treatment of gouty arthritis with an acute attack. International Journal of Rheumatic Diseases 2018;21(Suppl 1):218.
References to studies awaiting assessment
Wu 2014 {published data only}
-
- Wu YQ, Hu JH, Zhao ZY, Zhou C, Yu X, Zheng Y, et al. Clinical observation on senile patients with acute gouty arthritis treated by acupoint application. Journal of the American Geriatrics Society 2014;62(Suppl 2):S381.
References to ongoing studies
TCTR20180608001 {published data only}
-
- TCTR20180608001. Comparing efficacy of loading and non-loading colchicine in acute crystal induced arthritis. www.thaiclinicaltrials.org 7 June 2018.
Additional references
Agudelo 1972
-
- Agudelo CA, Schumacher HR Jr, Phelps P. Effect of exercise on urate crystal-induced inflammation in canine joints. Arthritis and Rheumatism 1972;15:609-16. - PubMed
Araújo 2015
-
- Araújo F, Cordeiro I, Ramiro S, Falzon L, Branco JC, Buchbinder R. Outcomes assessed in trials of gout and accordance with OMERACT-proposed domains: a systematic literature review. Rheumatology 2015;54(6):981-93. - PubMed
Arnold 1988
Australian Rheumatology Association 2019
-
- Australian Rheumatology Association. Patient information on colchicine (last updated April 2019). rheumatology.org.au/patients/documents/Colchicine_2019v2.pdf (accessed 16 November 2020).
Bellamy 1987
Ben‐Chetrit 1998
-
- Ben-Chetrit E, Levy M. Colchicine: 1998 update. Seminars in Arthritis and Rheumatism 1998;28:48-59. - PubMed
Boutron 2008
-
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, CONSORT Group. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine 2008;148:295-309. - PubMed
Cates 2008 [Computer program]
-
- Visual Rx. Version 3. Dr. Chris Cates' EBM website, 2008. www.nntonline.net.
Covidence 2021 [Computer program]
-
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 27 August 2020. Available at covidence.org.
Cox 2004
Dalbeth 2014
De Angelis 2004
-
- De Angelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical Trial Registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292(11):1363-4. - PubMed
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Dorwart 1974
-
- Dorwart BB, Hansell JR, Schumacher HR Jr. Effects of cold and heat on urate-induced synovitis in the dog. Arthritis and Rheumatism 1974;17(5):563-71. - PubMed
FDA 2008
-
- Food and Drug Administration. Drug products containing colchicine for injection; enforcement action dates. Federal Register (updated February 2008). Department of Health and Human Services, 2008. Available at www.federalregister.gov/documents/2008/02/08/08-564/drug-products-contai....
FitzGerald 2020
GRADEpro 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 8 September 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hak 2010
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021b
-
- Higgins JP, Eldridge S. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hill 1965
Hui 2017
-
- Hui M, Carr A, Cameron S, Davenport G, Doherty M, Forrester H, et al, British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology Guideline for the management of gout. Rheumatology 2017;56:e1-e20. - PubMed
Janssens 2008a
-
- Janssens HJ, Janssen M, Van de Lisdonk EH, Van Riel PL, Van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet 2008;371:1854-60. - PubMed
Janssens 2008b
Kasper 2005
-
- Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson DL, et al. Harrison's Principles of Internal Medicine. 16th edition. McGraw-Hill Professional, 2005.
Kuncl 1987
-
- Kuncl R, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. New England Journal of Medicine 1987;316(25):1562-8. - PubMed
Mikuls 2005
Moi 2013
Morris 2003
O'Sullivan 1972
RevMan 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Richette 2017
-
- Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Annals of the Rheumatic Diseases 2017;76:29-42. - PubMed
Schlesinger 2001
-
- Schlesinger N, Schumacher HR Jr. Gout: can management be improved? Current Opinion in Rheumatology 2001;13(3):240-4. - PubMed
Schlesinger 2004
-
- Schlesinger N. Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs 2004;64(21):2399-416. - PubMed
Schumacher 1993
-
- Schumacher H, Klippel J, Koopman W. Primer on the Rheumatic Diseases. 10th edition. Arthritis Foundation, 1993.
Schumacher 2005
-
- Schumacher HR, Edwards LN, Perez-Ruiz F, Becker M, Chen LX, Furst DE. Outcome measures for acute and chronic gout. Journal of Rheumatology 2005;32(12):2452-5. - PubMed
Schumacher 2009
-
- Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. Journal of Rheumatology 2009;36(10):2342-5. - PubMed
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Shekelle 2017
Singh 2011
Singh 2014
Sivagnanam 2004
Stewart 2020
Tugwell 2004
-
- Tugwell P, Shea B, Boers M, Brooks P, Simon LS, Strand V, et al. Evidence-Based Rheumatology. BMJ Books, 2004.
van der Heijde 2014
-
- Sivera F, Andrés M, Carmona L, Kydd AS, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of abroad panel of rheumatologists in the 3e initiative. Annals of the Rheumatic Diseases 2014;73:328–35. - PMC - PubMed
Wallace 1977
-
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu T-F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and Rheumatism 1977;20:895-900. - PubMed
Wallace 1998
-
- Wallace SL, Singer JZ. Therapy in gout. Rheumatic Diseases Clinics of North America 1988;14:441-57. - PubMed
Wechalekar 2013
Wortmann 2002
-
- Wortmann RL. Gout and hyperuricemia. Current Opinion in Rheumatology 2002;14:281-6. - PubMed
References to other published versions of this review
Schlesinger 1997
-
- Schlesinger N, Baker DDB, Schumacher R. Interventions for hyperuricemia gout.. Cochrane Database of Systematic Reviews 1997, Issue 3. Art. No: CD000462. [DOI: 10.1002/14651858.CD000462.pub2] - DOI
Schlesinger 2006
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

