Early Tumor Response and Safety of Atezolizumab Plus Bevacizumab for Patients with Unresectable Hepatocellular Carcinoma in Real-World Practice
- PMID: 34439111
- PMCID: PMC8394131
- DOI: 10.3390/cancers13163958
Early Tumor Response and Safety of Atezolizumab Plus Bevacizumab for Patients with Unresectable Hepatocellular Carcinoma in Real-World Practice
Abstract
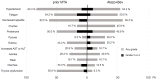
The aim of this study was to investigate the early tumor response and safety of atezolizumab plus bevacizumab for patients with unresectable hepatocellular carcinoma in real-world practice. Forty patients with Child-Pugh class A liver function and eastern cooperative oncology group performance status 0 or 1 were enrolled. The objective response rate (ORR) at six weeks after the start of treatment, changes in α-fetoprotein (AFP) and des-γ-carboxyprothrombin, incidence of adverse events (AEs), and changes in albumin-bilirubin (ALBI) score and serum ammonia level, were evaluated. Among 40 patients, 24 had histories of prior molecular targeted agents (MTAs). The ORR was 22.5% based on mRECIST. Multivariate analysis showed that an AFP ratio <1.0 at three weeks (odds ratio 39.2, 95% confidence interval CI 2.37-649.0, p = 0.0103) was the only significant factor for predicting early response. There was no significant difference in the frequency of AEs between patients receiving first-line treatments and others. Fatigue, proteinuria, and ascites were more frequent in patients who experienced prior treatment. No decrease in ALBI score or increase in serum ammonia level was observed. Our study demonstrated that AFP may be useful in assessing early response and that this treatment is safe, including in patients with prior MTA treatments.
Keywords: atezolizumab; bevacizumab; hepatocellular carcinoma; immune checkpoint inhibitor; molecular targeted agents; real-world practice; tumor marker; α-fetoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Herbst R.S., Soria J.-C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. - DOI - PMC - PubMed
-
- Finn R.S., Bentley G., Britten C.D., Amado R., Busuttil R.W. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009;29:284–290. doi: 10.1111/j.1478-3231.2008.01762.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

