The Renin-Angiotensin System in the Tumor Microenvironment of Glioblastoma
- PMID: 34439159
- PMCID: PMC8392691
- DOI: 10.3390/cancers13164004
The Renin-Angiotensin System in the Tumor Microenvironment of Glioblastoma
Abstract
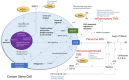
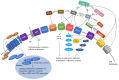
Glioblastoma (GB) is an aggressive primary brain tumor. Despite intensive research over the past 50 years, little advance has been made to improve the poor outcome, with an overall median survival of 14.6 months following standard treatment. Local recurrence is inevitable due to the quiescent cancer stem cells (CSCs) in GB that co-express stemness-associated markers and components of the renin-angiotensin system (RAS). The dynamic and heterogeneous tumor microenvironment (TME) plays a fundamental role in tumor development, progression, invasiveness, and therapy resistance. There is increasing evidence showing the critical role of the RAS in the TME influencing CSCs via its upstream and downstream pathways. Drugs that alter the hallmarks of cancer by modulating the RAS present a potential new therapeutic alternative or adjunct to conventional treatment of GB. Cerebral and GB organoids may offer a cost-effective method for evaluating the efficacy of RAS-modulating drugs on GB. We review the nexus between the GB TME, CSC niche, and the RAS, and propose re-purposed RAS-modulating drugs as a potential therapeutic alternative or adjunct to current standard therapy for GB.
Keywords: cancer stem cell niche; cancer stem cells; glioblastoma; organoids; pluripotent stem cells; renin–angiotensin system; tumor microenvironment.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. S.T.T. is an inventor of the patents Cancer Diagnosis and Therapy (PCT/NZ2015/050108; AUS/2012302419; JAP/2017528398; US/0281472), Cancer Therapeutic (PCT/NZ2018/050006), and Novel Pharmaceutical Compositions for Cancer Therapy (PCT/NZ2019/050087).
Figures
References
-
- Louis D.N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. - DOI - PMC - PubMed
-
- Wang Q., Hu B., Hu X., Kim H., Squatrito M., Scarpace L., DeCarvalho A.C., Lyu S., Li P., Li Y., et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell. 2017;32:42–56.e46. doi: 10.1016/j.ccell.2017.06.003. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

