Metformin Is a Pyridoxal-5'-phosphate (PLP)-Competitive Inhibitor of SHMT2
- PMID: 34439169
- PMCID: PMC8393646
- DOI: 10.3390/cancers13164009
Metformin Is a Pyridoxal-5'-phosphate (PLP)-Competitive Inhibitor of SHMT2
Abstract
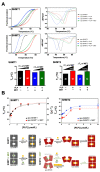
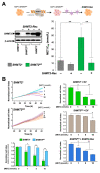
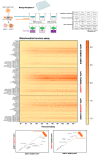
The anticancer actions of the biguanide metformin involve the functioning of the serine/glycine one-carbon metabolic network. We report that metformin directly and specifically targets the enzymatic activity of mitochondrial serine hydroxymethyltransferase (SHMT2). In vitro competitive binding assays with human recombinant SHMT1 and SHMT2 isoforms revealed that metformin preferentially inhibits SHMT2 activity by a non-catalytic mechanism. Computational docking coupled with molecular dynamics simulation predicted that metformin could occupy the cofactor pyridoxal-5'-phosphate (PLP) cavity and destabilize the formation of catalytically active SHMT2 oligomers. Differential scanning fluorimetry-based biophysical screening confirmed that metformin diminishes the capacity of PLP to promote the conversion of SHMT2 from an inactive, open state to a highly ordered, catalytically competent closed state. CRISPR/Cas9-based disruption of SHMT2, but not of SHMT1, prevented metformin from inhibiting total SHMT activity in cancer cell lines. Isotope tracing studies in SHMT1 knock-out cells confirmed that metformin decreased the SHMT2-channeled serine-to-formate flux and restricted the formate utilization in thymidylate synthesis upon overexpression of the metformin-unresponsive yeast equivalent of mitochondrial complex I (mCI). While maintaining its capacity to inhibit mitochondrial oxidative phosphorylation, metformin lost its cytotoxic and antiproliferative activity in SHMT2-null cancer cells unable to produce energy-rich NADH or FADH2 molecules from tricarboxylic acid cycle (TCA) metabolites. As currently available SHMT2 inhibitors have not yet reached the clinic, our current data establishing the structural and mechanistic bases of metformin as a small-molecule, PLP-competitive inhibitor of the SHMT2 activating oligomerization should benefit future discovery of biguanide skeleton-based novel SHMT2 inhibitors in cancer prevention and treatment.
Keywords: folate; glycine; one-carbon metabolism; serine; serine hydroxymethyltransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ducker G.S., Ghergurovich J.M., Mainolfi N., Suri V., Jeong S.K., Hsin-Jung Li S., Friedman A., Manfredi M.G., Gitai Z., Kim H., et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA. 2017;114:11404–11409. doi: 10.1073/pnas.1706617114. - DOI - PMC - PubMed
-
- Cuyàs E., Fernández-Arroyo S., Buxó M., Pernas S., Dorca J., Álvarez I., Martínez S., Pérez-Garcia J.M., Batista-López N., Rodríguez-Sánchez C.A., et al. Metformininduces a fasting- and antifolate-mimicking modification of systemic host metabolism in breast cancer patients. Aging. 2019;11:2874–2888. doi: 10.18632/aging.101960. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

